MRI safely performed in patients with cardiac devices long-considered to be incompatible
Magnetic resonance imaging exams can be safely performed in patients with cardiac devices previously considered incompatible with MRI machines, according to a study published Thursday in Radiology: Cardiothoracic Imaging.
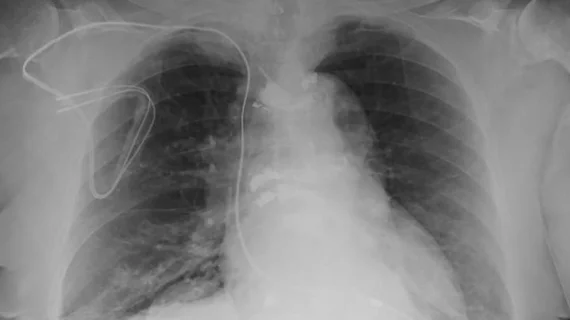
The U.S. Food and Drug Administration has labeled a subset of implanted devices as MR-conditional, meaning they are safe only in certain scenarios. In those with pacemakers and implantable cardioverter defibrillators (ICD) that don’t meet the FDA’s criteria, however, clinicians must either remove the equipment or choose another imaging test.
But after examining hundreds of patients according to a prespecified MRI protocol, the researchers reported no serious adverse events in non-MR conditional individuals.
It’s great news for the millions who rely on implanted devices such as pacemakers, but were prohibited from undergoing MRI exams, said lead author Sanjaya K. Gupta, MD, from Saint Luke's Mid America Heart Institute in Kansas City, Missouri.
“While all devices implanted in patients today are MRI compatible, millions of people worldwide, including many young people, have older devices considered not compatible," Gupta added in a statement. "It's unfair to tell these people that they can't get an MRI for the rest of their lives."
There has been past research confirming the safety of performing MRI exams in those with non-MR conditional devices, but those investigations didn’t include pacemaker-dependent ICD patients, those whose life relies on a defibrillator, and a number of other individuals.
With this in mind, Gupta et al. established the Patient Registry of Magnetic Resonance Imaging in Non-Approved Devices, or PROMeNADe. The registry comprises more than 500 people who received 608 MRI exams, including 61 cardiac MRIs.
As part of the investigation, patients’ devices and vital signs were closely monitored. Gupta and colleagues determined that MRI testing, including chest exams, can be safely completed in pacemaker-dependent ICD patients and those with non-MR conditional devices or abandoned leads.
"The protocol worked amazingly well. We had no issues with any of the patients and no harm to the devices,” Gupta added. “We're hopeful that our work will add support to expand the FDA's indications for devices that are considered MRI-compatible.”
You can read the entire study here.