Multimodality imaging turns up serial thromboses following AstraZeneca COVID vaccination
A new case series published June 14 in Radiology details multi-system thrombosis in patients with reactive thrombocytopaenia after receiving their first dose of AstraZeneca's COVID vaccine.
The study analyzed the cases of 40 patients who developed symptoms of thrombocytopaenia and thrombosis shortly after receiving their first dose of the COVID vaccine—80% within 14 days and 20% within 28 days. Of these patients, 73% experienced neurological symptoms and 20% died. Concerningly, the diagnoses were undetectable via standard clinical methods alone and were determined with whole-body imaging using multiple modalities.
“International guidance suggests symptom-specific imaging for vaccine-induced immune thrombotic thrombocytopenia, but there remains a paucity of data on the prevalence of multi-site thrombosis and the implications of this on management,” corresponding author Conrad Von Stempel, of University College London Hospital, and co-authors shared. “This is the largest study to date of patients with vaccine-induced immune thrombotic thrombocytopenia with whole-body imaging and multi-system thrombosis.”
The authors explain that the guidance implies imaging of this specific cohort of patients should be targeted only to localizing symptoms. Concerned with the high probability of multi-site thrombosis in these patients, the researchers sought to investigate the utility of whole-body imaging to assess the frequency and location of thrombosis in each vascular system. They achieved this by analyzing the CT, MRI and ultrasound exams and reports on a UK-wide cohort of patients with confirmed vaccine-induced immune thrombotic thrombocytopenia (VITT).
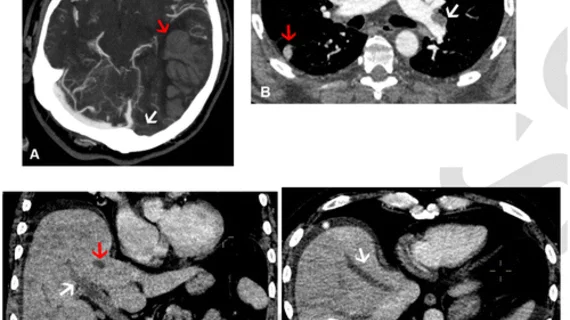
Through this assessment, the experts identified the following:
a combination of cerebral venous sinus thrombosis and pulmonary embolism in 16 of 40 patients (40%)
cerebral venous sinus thrombosis and portomesenteric venous thrombosis in 9 of 40 patients (23%)
34 of 40 with cerebral venous sinus thrombosis
17 of 40 with pulmonary embolism
10 of 40 with portomesenteric venous thrombosis
11 of 40 with deep venous thrombosis
8 of 40 with systemic arterial thrombosis
Out of the 40 patients included in the research, 25 had additional imaging completed during the study, 83% of whom had additional sites of thrombosis identified. Authors of the study suggested that these findings further confirm the utility of whole-body imaging when patients present with VITT.
“This is much higher than the reported diagnoses of VITT where multi-site thrombosis was seen in 27-50% of patients, likely reflecting the fact that many of these studies did not perform comprehensive whole- body imaging,” the experts explained. “These findings emphasize that VITT is a multisystem disorder and suggest that whole-body contrast-enhanced imaging is likely to identify further thrombosis.”
View the detailed research here.
References:
Thrombus Distribution in Vaccine-induced Immune Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. Priya Rogers, Ieuan Walker, Jason Yeung, Abeera Khan, Anmol Gangi, Behnaz Mobashwera, Robert Ayto, Ali Shah, Joannes Hermans, Andrew Murchison, Matthew Benger, Sean Apap Mangion, Puja R Mehta, Laszlo Sztriha, Simrit Ghatorae, Brian Craven, Marie Scully, Timothy Bray, Margaret Hall–Craggs, Conrad Von Stempel, and “for the RADIANT Group”. Radiology.
Related COVID vaccine coverage:
New research compares COVID pneumonia in vaccinated vs unvaccinated patients
Reactive lymphadenopathy slower to resolve after Moderna COVID vaccination
New cardiac MRI analysis offers updated insight into long-term impact of vaccine-related myocarditis
Experts suggest new follow-up imaging protocols for vaccine-related lymphadenopathy