Reactive lymphadenopathy slower to resolve after Moderna COVID vaccination
New research highlights key factors associated with the resolution of reactive lymphadenopathy visualized on breast imaging after patients receive an mRNA COVID vaccine.
“The variable clinical course of subclinical lymphadenopathy detected on breast imaging after COVID-19 vaccination creates management challenges and has led to evolving practice recommendations,” corresponding author Michele B. Drotman, MD, from the Department of Radiology at Weill Cornell Medicine, and co-authors explained.
Vaccine-related lymphadenopathy is a well-documented side effect of mRNA COVID vaccines but, until recently, little was known about the specifics pertaining to its clinical course and resolution. A new study published in the American Journal of Roentgenology this week confirmed prior findings that indicated the reactive inflammation is benign in nature and can take weeks to resolve, and also took the research a step further by characterizing features that are associated with the amount of time it takes for the side effect to fully subside.
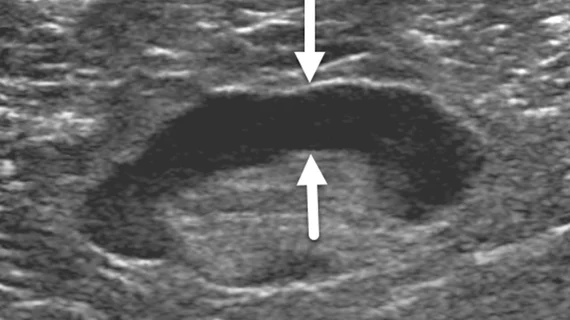
This involved retrospectively assessing 111 women who experienced axillary lymphadenopathy ipsilateral to either a Pfizer or Moderna COVID vaccine. After the findings were initially detected via ultrasound (within 8 weeks of inoculation), the women underwent follow-up ultrasound imaging in 4 to 12 week intervals until the lymphadenopathy resolved.
The researchers found the reactive inflammation subsided 127±43 days after the first vaccine dose and 97±44 days after the initial ultrasound. Significant associations between shorter resolution times and Pfizer vaccinations were observed (compared to Moderna vaccination) in addition to longer resolution times for the women who received their second vaccine dose after having already developed lymphadenopathy.
Imaging features were also examined as predictive factors, with cortical thickness of the nodes being of particular interest. Greater cortical thickness observed on initial imaging was found to be an independent predictor of lengthier resolution times.
Patient factors, such as age, prior history of breast cancer, and axillary symptoms did not appear to be associated with time to resolution.
“Axillary lymphadenopathy detected by breast ultrasound after COVID-19 mRNA vaccination lasts longer than reported in initial vaccine clinical trials,” the author wrote. “The prolonged time for resolution supports not delaying screening mammography due to recent COVID-19 vaccination as well as the recent professional society recommendation of a follow-up interval of at least 12 weeks for suspected vaccine-related lymphadenopathy.”
More on vaccine-related lymphadenopathy:
Experts suggest new follow-up imaging protocols for vaccine-related lymphadenopathy
These ultrasound features distinguish between COVID vaccine-related and malignant adenopathy
Mammograms should not be delayed after COVID vaccine, research shows
References:
“Time for Resolution of COVID-19 Vaccine-Related Lymphadenopathy and Associated Factors,” Elizabeth G. Lane, Carolyn S. Eisen, Michele B. Drotman, Katerina Dodelzon, Eralda Mema, Charlene Thomas, and Martin R. Prince, American Journal of Roentgenology.