FDA clears mobile 1.5T MRI system
For patients in rural areas who may not have access to MRI services, the U.S. Food and Drug Administration (FDA) has opened the door for one to come to you.
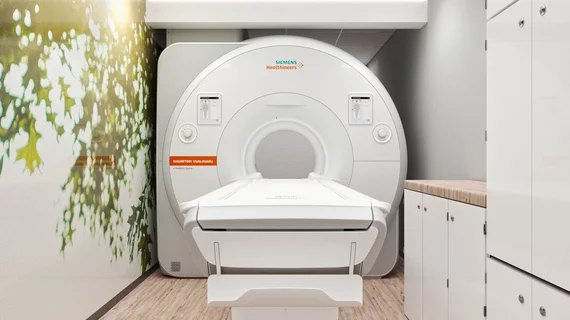
Siemens Healthineers’s MAGNETOM Viato.Mobile 1.5 Tesla MRI, announced late last year, has received 510(k) clearance from the FDA. The machine is designed to be transported to an off-site location, effectively providing access to MRI scans from anywhere.
The MAGNETOM Viato.Mobile system is contained in a trailer that can be parked at hospitals, health systems, or clinics either as an additional machine for overloaded radiology units or as a dedicated MRI system for facilities that may not have the budget or space to build the necessary shielded room.
According to a statement from Siemens sent to Health Imaging, the mobile scanner offers some of the same features of larger systems, including AI to accelerate brain scans, reduce noise and artifacts, and ultimately improve image resolution. There is also technology that Siemens says helps the system to adapt to the physiology of a patient, to personalize a scan for better image results.
There is also an on-board guidance system to assist radiology technicians in performing a scan, catered to their experience level. This is useful for areas that may have trained radiologists who don’t get to perform MRI scans very often due to a lack of access to a fixed unit.
In the statement announcing the FDA clearance, Jane Kilkenny, vice president of magnetic resonance at Siemens Healthineers, said the company is dedicated to mobile imaging and providing people, regardless of location, access to the latest technology.
“The introduction of the MAGNETOM Viato.Mobile is yet another example of our efforts to democratize high-end imaging technology to provide greater access to care,” she said.
Siemens received FDA clearance for other models of its MAGNETOM MRI system in 2021 and 2022.