Automated imaging locks in on brain proteins to detect earliest signs of Alzheimer’s disease
Many consider amyloid-beta and tau protein deposition as the catalysts for Alzheimer’s disease, yet tracking their accumulation early in the process remains a challenge. But researchers from one well-known Boston institution may have an answer.
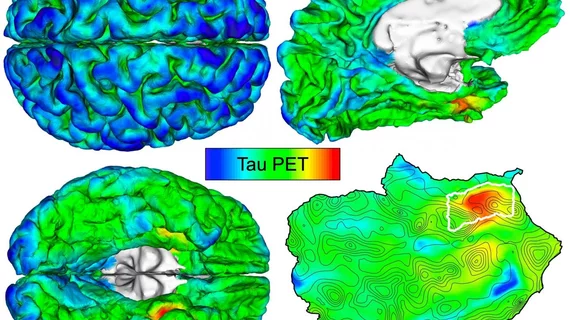
Massachusetts General Hospital researchers trained their automated imaging method on hundreds of PET scans and shared their findings recently in Science Translational Medicine. They pinpointed where tau first originated and noted patients with the highest initial levels of deposition went on to experience the most spread throughout their entire brain.
With further testing, it may be possible to gather PET measurements of tau in specific brain regions to inform future treatments and potentially predict a patient’s risk of developing the deadly disease earlier.
"While our understanding of Alzheimer's disease has increased greatly in recent years, many attempts to treat the condition so far have failed, possibly because medical interventions have taken place after the stage at which the brain injury becomes irreversible," lead author Justin Sanchez, a data analyst at MGH's Gordon Center for Medical Imaging, said in a statement.
To develop their automated approach, Sanchez et al. evaluated PET brain scans of amyloid-beta and tau from 443 patients participating in aging and Alzheimer’s disease studies. Individuals ranged from healthy 20-year-olds to adults with clinically diagnosed Alzheimer’s dementia.
The MGH team determined that tau is initially deposited in the rhinal cortex region of the brain before it spreads to the temporal neocortex. And they were able to observe this phenomenon in cognitively normal patients with zero evidence of elevated amyloid-beta, they explained.
Furthermore, after following 104 participants for two years, the researchers determined those with the highest initial levels of tau suffered the most spread throughout their brain over time.
The bottom line: using PET measurements of tau proteins could determine a patient’s future risk of Alzheimer’s disease and, if caught early, potentially prevent it altogether.
“Clinical trials evaluating the efficacy of anti-tau therapeutics would benefit from an automated, individualized imaging method to select cognitively normal individuals vulnerable to impending tau spread, thus advancing our efforts to provide effective interventions for patients at risk for Alzheimer's disease," Sanchez concluded