PSMA-PET is rapidly changing the standard of care for prostate cancer patients
A new imaging technology that has seen rapid growth and may be a paradigm shift in prostate cancer care has come about with the arrival of new prostate-specific membrane antigen (PSMA) targeted radiotracers used in positron emission tomography (PET) scans. Since the first U.S. approval of a PSMA-PET imaging agent in 2020 (followed by more agents in 2021 and 2022) the technology has become a key topic of discussion at oncology, radiology, molecular imaging and radiation therapy conferences.
Adoption of PSMA-PET has been swift because it can significantly improve prostate cancer detection and treatment. Prostate cancer tumors over-express PSMA proteins, making it so that PSMA radiotracers bind to them more readily. This makes the prostate cancer much easier to visualize. This includes even very small levels of tumor cells that have metastasized to other areas of the body and are often missed with conventional prostate imaging.
"It is an exciting new technology that is in many ways changing the way prostate cancer is managed," explained Munir Ghesani, MD, FACNM, FACR, President of the Society of Nuclear Medicine and Molecular Imaging (SNMMI), System Chief of Nuclear Medicine at Mount Sinai Health, and Associate Professor of Radiology at Icahn School of Medicine at Mount Sinai. "I can say without any hesitation the literature that will evolve from the widespread use of PSMA-PET in the coming years is going to result in a quite different management algorithm for prostate cancer in the future."
Ghesani said PSMA-PET has revolutionized prostate cancer care because it can see very tiny amounts of cancer cells that otherwise would have been missed, but it also helps catch and treat areas of cancer beyond the prostate bed. He also explained radiation oncologists can use the technology to zero in on metastases. The imaging makes it much easier to contour images and ensure areas of small tumors are included in the treatment plan to improve patient outcomes.
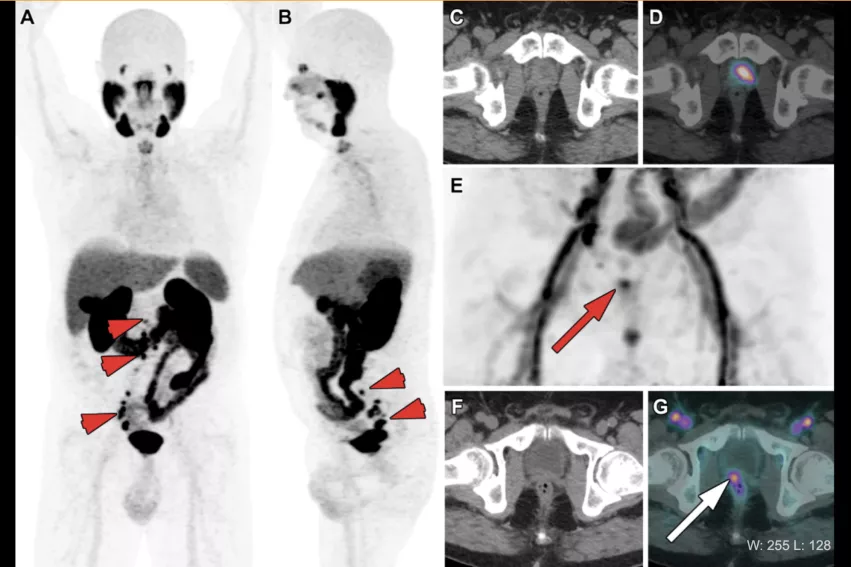
PSMA-PET example of biochemical recurrence of prostrate (PSA level = 1.1 ng/mL) in a 72-year-old patient 3 years after radical prostatectomy, chemotherapy and ADT for metastatic Gleason score 9 (5 + 4) prostate cancer. (A, B) Anterior MIP images from 68Ga-PSMA PET show multiple pelvic and retroperitoneal lymph node metastases (short arrows) and a sternal bone lesion (dashed arrow). There is also intense PSMA uptake (SUVmax [maximum standardized uptake value] = 45.0) in a large typical local recurrence on the left side of the prostate bed (arrowhead in B). Depending on PET windowing parameters, it may be difficult to differentiate that lesion from urinary activity (A), yet it becomes clearly visible after adjusting PET windows (arrowhead in B–D). (E–G) Acquisition of early PET images is another way to enhance detection of local recurrence (arrowhead), taking advantage of the absence of urinary activity at this time point, thus preventing urine from hiding neoplastic tissue. See more examples in this RSNA article. Image Courtesy of RSNA
The impact of PSMA-PET on biochemical recurrence of prostate cancer
Ghesani said PSMA-PET has had a major impact on biochemical recurrence of prostate cancer, which has traditionally been detected using PSA tests, tracking elevated levels over time. But the issue has been finding where the cancer lurks when it does not appear on imaging because the tumor cells are below the threshold where they are visible on conventional medical imaging.
"It left little doubt that there was a recurrence of cancer, but if you used traditional imaging, including CT, MRI and bone scans, and you cannot find the cancer, you have to manage the patient very arbitrarily. But now that we have the PSMA-PET available, we now often see the cancer in concentrated in very small lymph nodes that are only 2 or 3 mm in size. Those lymph nodes were not suspicious enough on traditional imaging sequences to call them positive. Now that we know that even a small amount of tissue can be harboring the cancer, you can do a specifically targeted treatment."
He said this has led to a lot of excitement in both the medical imaging and the radiation oncology communities because they now have a tool to better detect and treat the cancer.
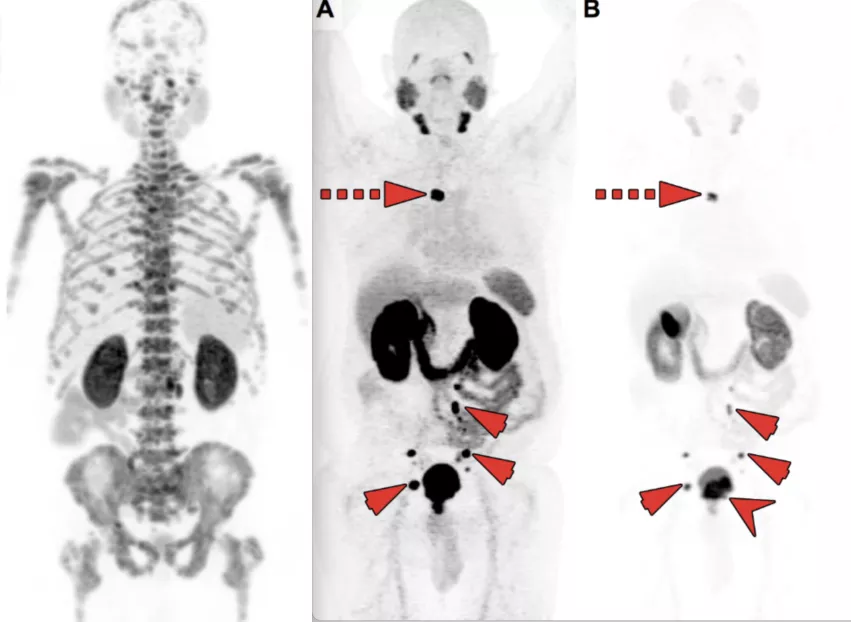
Two cases of PSMA-PET imaging showing tumors outside of the traditional pelvic bed where they are usually found in patients with chemical reoccurrence of prostate cancer. Several centers now widen their field of view when imaging to catch tumors that may have been missed in the past and often did not appear at all using imaging techniques other than PSMA-PET. Left image shows extensive bone metastasis. Image courtesy of SNMMI. Right image shows a chest tumor far above the typical pelvic tumors. Image courtesy of RSNA. Find more clinical examples.
PSMA-PET found new patterns for the spread of prostate cancer
PSMA-PET also has shown that biochemical recurrence of prostate cancer does not always spread in a systematic way, sometimes showing up in areas of the body where no one was looking. This has changed how some centers conduct imaging, widening their field of view to including more parts of the body.
"Prior to widespread use of these agents, it was believed that whenever the cancer spreads it usually spreads through the venous plexus, going up through the channels, so there is an expected pattern of spread," Ghesani said. "But in a small minority of cases, it skips all of those areas and we find very unusual sites for the disease, such as the chest for example, which are areas you traditionally would not scan."
Typically, he said imaging for prostate cancer only include the abdomen and pelvis. His center now includes expanding the field of view to include the chest.
At tumor boards where these findings were first shown, the oncologists biopsied the lung lesions detected by PSMA-PET and confirmed this was metastatic prostate cancer.
"They would then always make it a point telling me that if it was not PSMA-PET/CT, we never would have known the cancer had spread outside of the traditional route of the spread of the disease," he said.
Costs and time might be reduced by eliminating other exams and just using PSMA-PET
The ability of PSMA-PET to clearly see prostate cancer and its spread to other parts of the body may change the current workflow and reduce the need for extra exams.
"We are still in the transition stage to PSMA-PET, but I can see the evolving pattern for patient workups, that it may in many instances eliminate the need for a CT scan and a bone scan first before a PSMA-PET exam," Ghesani explained.
How many PSMA-PET radiotracers are on the U.S. market?
Currently there are four PSMA-PET targeted radiotracer agents and one nuclear medicine PSMA targeted therapy that are cleared by the U.S. Food and Drug Administration (FDA). Three are gallium (Ga)-based agents and one is a fluorine (F) based radiotracer. The PSMA target therapy agent is a lutetium (Lu)-based radiotherapy agent. These agents include:
• Gallium 68 PSMA-11 (Ga 68 PSMA-11), FDA granted approval to the University of California, Los Angeles and the University of California, San Francisco, in December 2020.
• Pylarify (piflufolastat F-18, or 18F-DCFPyL), Lantheus Medical Imaging and Progenics Pharmaceuticals, Inc., cleared in May 2021.
• Illuccix (68Ga-PSMA-11, 68Ga-gozetotide), Telix Pharmaceuticals Ltd. and Novartis, cleared by the FDA in December 2021.
• Locametz (gallium Ga 68 gozetotide), Advanced Accelerator Applications USA, Inc., Novartis, cleared by the FDA in March 2022. It was cleared as an indication for selection of patients with metastatic prostate cancer, for whom lutetium Lu-177 vipivotide tetraxetan PSMA-targeted therapy can be used.
• Pluvicto (177Lu-vipivotide tetraxetan, or 177Lu-PSMA-617), Novartis and Advanced Accelerator Applications USA. It is a radiopharmaceutical therapy (RPT) to treat prostate cancer. It was approved the same day as Locametz, which is used in the patient selection of the therapy.
Another agent that is discussed in online articles, at meetings and compared to PSMA-PET agents is the radiotracer Axumin (fluciclovine F-18) from Blue Earth Diagnostics. It was cleared by the FDA in 2016 for restaging of prostate cancer. PSMA PET scans are FDA approved for both initial staging of prostate cancer for men recently diagnosed with certain types of higher risk cancers, as well as for restaging men with suspected recurrent prostate cancer. Head-to-head studies of the Axumin vs. PSMA-PET have found PSMA-PET is more accurate. [1-3]
Reimbursement for PSMA-PET nuclear imaging
As of January 1, 2022, the Centers for Medicare and Medicaid Services (CMS) have provided PSMA-PET scans with a dedicated billing code, which in turn facilitated Medicare creating a consistent reimbursement schedule, making PSMA-PET scans reimbursable. More details can be found under Group 10 codes in the Billing and Coding: Positron Emission Tomography Scans Coverage on the CMS website.[3]
It is widely expected that as clinical evidence continues to mount that private health insurance companies will also begin to reimburse for PSMA-PET.
Editor's Note: Ghesani's primary research interests are in clinical and translational areas in oncologic imaging and targeted radionuclide therapies. This includes working with the FDA, where he has helped successfully introduce novel research radiotracers into clinical use. Ghesani serves in leadership roles in various societies and colleges, including as the SNMMI president, past chairman of the American Board of Nuclear Medicine, past chairman of the Government Relations Committee and the FDA Task Force of the SNMMI and past chairman of the Nuclear Medicine and PET Accreditation Committee of the American College of Radiology (ACR). In 2006, he was selected as the Fellow of the American College of Nuclear Medicine and in 2018 received the fellowship of the ACR.