Radiotracers on FDA’s fast track exceed performance of already approved tau-detecting agent
Two new radiotracers not yet approved for use in the U.S. just outperformed an FDA approved agent used to detect tau tangles in the brain.
The presence of tau tangles is a telltale sign of Alzheimer’s. These abnormal clusters of protein form in the brain’s neurons and have been implicated in cognitive impairments, with more tangles correlating with greater cognitive dysfunction.
Identifying and quantifying these tangles on imaging is a cornerstone in the diagnosis and management of Alzheimer’s disease and treatment. This is achieved with the help of radiotracers that, in effect, illuminate the tangles on PET imaging.
18F-Flortaucipir, also known by its brand name TAUVID, is approved by the U.S. Food and Drug Administration for use in PET imaging to identify tau tangles. Recently, experts compared the tracer’s utility alongside two other next-generation agents—18F-MK6240 and 18F-PI2620—that have not yet been approved. (They have been given the agency’s fast track designation.) The team determined that the two currently unauthorized agents could offer providers even greater insight into the process of patients’ neurodegeneration.
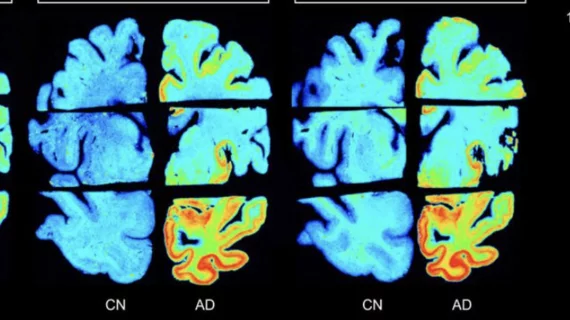
For the study, the team used autoradiography to assess the binding patterns of each tracer to two sample groups—one with autopsy-confirmed Alzheimer’s and another with healthy brain tissue. Binding values were then calculated for the prefrontal cortex, hippocampus and cerebellar cortex, as well as the whole-brain hemisphere.
All the tracers produced significant binding differences between healthy and diseased tissue in every brain region studied except for the cerebellar cortex. However, both 18F-MK6240 and 18F-PI2620 yielded higher binding values than the already approved TAUVID. What’s more, the two newer tracers also improved selectivity.
Experts involved in the research suggested that, once approved for clinical use, the two agents could improve Alzheimer’s care in the future.
“With their higher specificity, these new tau imaging agents are ideal for detecting the small changes that occur in brain tissue over time,” Eduardo R. Zimmer, PhD, assistant professor of pharmacology at the Federal University of Rio Grande do Sul, in Porto Alegre, Brazil, and colleagues noted. “This can be especially helpful in Alzheimer’s disease clinical trials that utilize tau PET as an outcome measure.”
Learn more about the findings in the Journal of Nuclear Medicine.