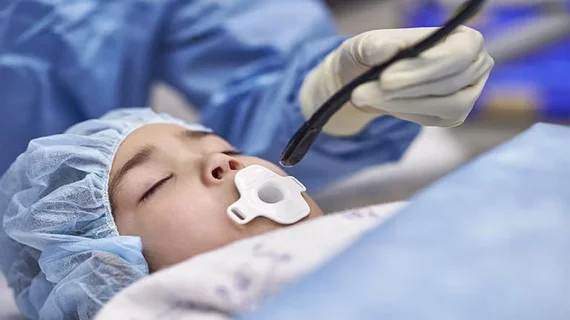
FDA clears mini transducer capable of creating 3D ultrasound images
The U.S. Food and Drug Administration (FDA) has cleared a mini transesophageal echocardiography (TEE) transducer, capable of producing high-quality 3D ultrasound images of the heart.
The X11-4t Mini from Philips Healthcare is 35% smaller than traditional models, designed to allow for easier cardiac scans with less strain on operators without sacrificing quality. The transducer is especially useful for pediatric care.
The smaller design makes 3D images possible in complex care situations where typically only 2D options were available because of the size of the transducer.
“That’s why we’ve developed a new, even smaller mini 3D TEE transducer that can be used to help physicians serve a wider range of patients, from small children to fragile adults,” David Handler, the vice president and general manager of Philips cardiology ultrasound added in a statement. “With this innovation we can help reduce the need for general anesthesia and lower the risk of complications, meaning patients may recover faster from procedures and can be discharged sooner.”
The mini device will seamlessly integrate into existing Philips EPIQ cardiac ultrasound systems, and it’s set to be commercially available in the U.S. this year. Approval in Europe is pending.