GBCA doses can be reduced by over 50% for some MRI exams
A new brain imaging study reveals that gadolinium-based contrast agent (GBCA) doses can be substantially reduced without risking image quality.
Safety concerns regarding the use of GBCAs have been highly debated for years. These conversations have led to numerous efforts to either develop contrast agents with less gadolinium in them (a collaboration between Bracco Diagnostics and Guerbet achieved this in 2022) or reduce the amount of contrast needed to acquire diagnostic quality MR imaging.
Research on the latter recently revealed that GBCA doses can be reduced by as much as 62% and still provide quality imaging of meningiomas. Details of the study were published this month in the American Journal of Neuroradiology.
“Gadolinium-based contrast agents are widely used for meningioma imaging; however, concerns exist regarding their side effects, cost, and environmental impact,” Tshea Dowers, MD, of the University of Toronto in Canada, and colleagues explain. “At the standard gadolinium dose, most meningiomas show avid contrast enhancement suggesting that administering a smaller dose may be feasible.”
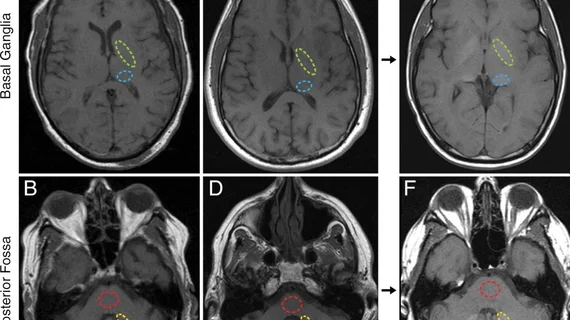
For the study, experts analyzed the imaging of 108 patients with confirmed or suspected meningiomas. Each patient underwent a contrast enhanced brain MRI, either using the standard GBCA dose, a low dose (approximately 62% less than the standard) or a micro dose (25% of the standard). Multiple readers analyzed the exams for quality and calculated the relative signal differences between the studies.
Both low and micro doses resulted in a statistically significant decrease in signal difference between tumors and their surrounding tissue. The low dose, however, yielded noninferior results compared to the standard dose, providing acceptable differentiation between meningiomas and the adjacent tissue.
“Reducing the gadolinium dose to 62% of the standard level still allows for sufficient visual delineation of meningiomas from surrounding tissues,” the group writes. “However, further reduction to 25% substantially compromises the ability to distinguish the tumor from adjacent structures.”
The authors suggest that although the micro dose is “not advisable,” the low dose protocol is clinically feasible.
The study abstract is available here.