Breast density notification laws: How will the FDA's updates affect radiologists and referring providers?
Earlier in March, the U.S. Food and Drug Administration released updated regulations pertaining to breast density notifications, which now require that facilities notify women of their breast density status following a mammogram.
Updates to the Mammography Quality Standards Act (MQSA) of 1992 were applauded by advocates who have been pushing for such changes in breast density reporting for years, but the changes also left some wondering what comes next.
To get a better idea of how these latest updates will affect patients, referring providers and radiologists, and what each group can do to prepare for the day the changes take hold, Health Imaging spoke with two individuals who are deeply rooted in the women’s imaging community—JoAnn Pushkin, executive director of DenseBreast-info.org and Stamatia Destounis, MD, managing partner of Elizabeth Wende Breast Care and chair of the ACR Breast Imaging Commission.
What do the updates mean for breast density notification requirements?
To understand how the updates might impact providers and patients, it’s important to first understand exactly what has changed.
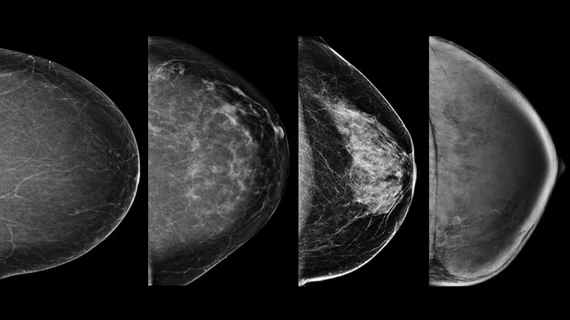
Starting in September 2024, the FDA will require that facilities notify patients of their breast density status—whether they are dense or not dense—following a mammogram. What this means for the patient must be explained in lay language that is easy to understand (the FDA has provided specific language for this). The new amendments also require that radiologists classify patients’ breast density using one of the four BI-RADS categories on imaging reports.
Facilities that do not comply could be subject to a citation from the FDA.
While most states have some form of density notification laws in place, these requirements vary widely, and some populous states—Connecticut, Louisiana, Maryland, Missouri, New Jersey, Texas—do not require that women be notified at all if they have dense breast tissue.
Given the lack of continuity to date, the FDA’s updated density notification requirements could spur an onslaught of questions not only from patients but also from referrers.
What’s next?
JoAnn Pushkin had her own breast cancer initially overlooked due to the masking effect of dense breast tissue. As a breast cancer survivor, she is an authoritative advocate for laws requiring providers to notify patients of the status of their breast density. In fact, she has spent years urging the FDA to introduce and implement a single nationwide breast density reporting standard.
Now that such a standard appears close at hand, Pushkin believes that what comes next will likely vary based on individual states and how stringent their prior notification requirements have been.
Since the FDA updates were announced, she has seen an increase in the number of women seeking information on supplemental screening.
“Based on the comments posted on news stories as well as those sent into the Contact Us page on the DenseBreast-info.org website, most of the questions relate to insurance coverage for supplemental screening,” she told Health Imaging. “Trying to determine levels of coverage or challenge a denial are frequent topics.”
Dr. Destounis also foresees an uptick in individuals seeking information on supplementary breast imaging in states that do not currently have detailed inform laws in place. She expects widespread change to develop slowly.
“It may take some time before we see a change upward toward supplemental screening. In states where women have to incur the cost of additional screening, no significant change may happen until the law includes insurance coverage,” Destounis said.
The Affordable Care Act mandates that insurance companies cover screening mammograms, which eliminates out-of-pocket costs for the exam. However, that coverage does not extend to supplemental screening for most women. For women with dense breasts, this lack of coverage could be especially consequential, as they are frequently advised to undergo additional imaging with modalities such as breast MRI.
Currently, 15 states and the District of Columbia have laws in place that require insurance coverage for supplemental screening, but the exams could still be subject to deductibles and co-pays. Multiple studies have shown women are more likely to decline supplementary imaging if it would result in additional OOP costs.
Pushkin has been working with Congresswoman Rosa DeLauro (D-Conn.), lending her support in the development of a federal law that would require insurance providers to offer no-cost coverage of breast cancer screening, supplementary or otherwise. The federal insurance bill, now known as the Find It Early Act, was introduced to Congress in December of 2022.
Pushkin points out the bill would require coverage extend to supplemental screening for women with dense breasts and for those who are at higher risk for developing breast cancer in accordance with National Comprehensive Cancer Network guidelines or American College of Radiology Appropriateness Criteria.
Until then, other concerns must be addressed before the FDA’s density notification regulations take effect. These include referring providers’ lack of understanding around breast density and how it affects a woman’s breast cancer risk.
How referring providers can prepare for patients’ questions about breast density and how radiologists can help
Prior studies have shown that many providers are underinformed on the ramifications of increased breast density. A recent study published in the Journal of Breast Imaging found that close to half of referring providers involved in the analysis did not know which risk model should be used to identify women who meet high-risk criteria for MRI screening.
Similarly concerning was a DenseBreast-Info.org study that found up to one-third of physicians and technologists overestimate the ability of digital breast tomosynthesis for detecting cancer in dense breasts as nearly equal to breast MRI.
In states that do not have inform laws, Destounis maintains, breast radiologists can be relied on as go-to experts, educating their staff on how to handle questions relating to breast density from patients and providers alike. She also names the American College of Radiology, Society of Breast Imaging and DenseBreast-Info.org as resources with ample guidance on the topic.
Destounis’s firsthand experience is clearly a reliable resource in its own right.
“In 2013 when the law in New York State came through, we reached out to our local medical society and brought a group together that included primary care physicians, business leaders, pediatricians, radiologists, patient navigators and surgeons. That helped us all understand what was in front of us,” she said. “We created presentations for the general healthcare providers to introduce this concept.”
Destounis offers three suggestions for referring providers preparing for an increase of questions relative to breast density:
Educate staff now with PowerPoint presentations, handouts and other materials on the topic that are easy to understand (available via ACR, SBI, DBI, etc.).
Create bullet points for your staff that are accessible during their daily shifts so that they can access them when questions arise.
Read up on the current literature to be able to discuss masking and other risks associated with increased breast density.
Destounis further recommends keeping stakeholders current on breast density news via quarterly electronic communications and newsletters. These can also be shared with patients either electronically or via pamphlets in waiting rooms, she adds.
The FDA’s updates require that patients’ breast density be reported to their referring providers using BI-RADS categories. Destounis suggests referring providers add educational materials to help explain these categories to patients. Ideally these would use photos or illustrations to show what each density category looks like on imaging as a way to help patients better understand their results.
Pushkin shared similar advice while adding that referring providers need to start preparing as soon as possible to have conversations about risks associated with breast density, in addition to learning how to appropriately facilitate orders for supplemental screening.
“Providers should be prepared to either be the source of information or be able to provide medically sourced resources,” Pushkin said.
Breast density resources for providers and patients
The changes are set to go into effect on September 10, 2024.
Resources with ample information on breast density and its associated risks, supplementary screening and photo examples of the four BI-RADS density categories can be found below:
The FDA’s updates to the Mammography Quality Standards Act (MQSA) of 1992 can be found here.