Targeted radionuclide alpha therapy study wins SNMMI's Abstract of the Year
The Society of Nuclear Medicine and Molecular Imaging has designated its coveted abstract of the year. The top entrant touts the benefits of targeted radionuclide alpha therapy for patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
The announcement was made this week at SNMMI’s annual meeting in Vancouver. The Society’s leadership reviewed more than 1,000 submissions, but ultimately voted for Abstract 325: “A Phase II clinical study on 225Ac-DOTATATE Therapy in Advanced Stage Gastroenteropancreatic Neuroendocrine Tumor Patients.”
The phase 2 study revealed significant survival rates and displayed an acceptable toxicity profile, which lead experts to conclude that 225Ac-DOTATATE could be a good option for patients with GEP-NETs who have not had success with other forms of therapy.
GEP-NETs—found in the GI tract—are rare. But when they are detected, they are most often to the point of metastasis. At this point in disease progression targeted radionuclide therapy is most often the best course of treatment, the experts involved in the study shared. Until recently, little was known about the efficacy of targeted alpha radionuclide therapy, such as 225Ac-DOTATATE, in the treatment of these rare malignancies.
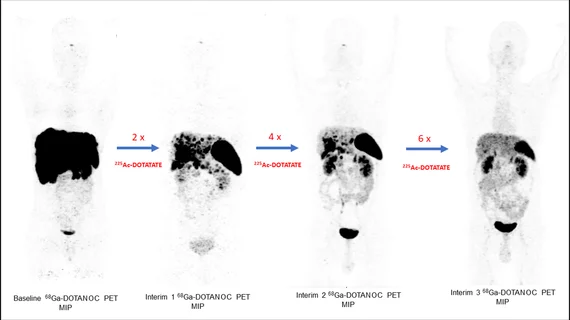
For their research, experts recruited 83 GEP-NET patients, each of whom had undergone previous therapy treatments prior to the study. After completing treatment with 225Ac-DOTATATE intravenously over 8 weekly intervals, the patients underwent a series of hematologic, kidney and liver function tests and had their tumor size and response/progression measured.
After treatment, 2.7% of patients had a complete response, followed by 43.2% with partial responses. Stable disease was noted in 34% and disease had progressed in 20%. Minimal toxicities occurred after treatment.
“225Ac-DOTATATE is a promising therapy option that adds a new dimension to the treatment of end-stage GEP-NETs, especially for patients who have tried all other standard therapy options,” said Chandrasekhar S. Bal, MD, professor and head of the Department of Nuclear Medicine and PET at the All India Institute of Medical Science in New Delhi, India. “These results warrant a Phase III randomized control trial to assess the true efficacy of 225Ac-DOTATATE versus 177Lu-DOTATATE.”
View SNMMI’s full release here.
Related radiation oncology news:
PSMA PET mapping improves radiation therapy contouring after PCA recurrence
Combination therapy that includes new radionuclide proves effective for metastatic prostate cancer
FDA approves new radioligand therapy for PSMA positive metastatic prostate cancer