Technology that creates interactive holograms of the heart cleared by FDA
Augmented reality is becoming more common in healthcare settings, with St. Louis-based developer SentiAR announcing it has received 510(k) clearance from the U.S. Food and Drug Administration, this time for its electrophysiology (EP) cardiac mapping system interface.
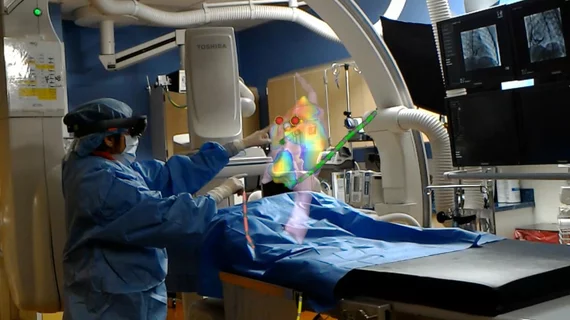
The CommandEP can integrate with holographic imaging systems to create 3D digital models of the heart in real time, providing physicians with an interactive, 360-degree map to use as a guide during surgery and other treatments, primarily cardiac ablation. The new interface allows the EP to view 3D mapping of the heart and manipulate the image with hand movements in the air during procedures so they do not break the sterile field or instruct other people how to move the image.
Much like with video games, augmented reality is made possible with a headset. A physician wears it and remains hands free to explore the holographic model of a patient's heart, viewing patient-specific anatomy details such as previous surgeries, catheter locations and abnormalities. This can be done without totally obscuring reality, allowing the holograms to be utilized at the tableside in the EP lab.
The system is currently in use at Massachusetts General Hospital, which commented in an statement sent out by SentiAR:
"Our initial clinical experience with the CommandEP system has been promising. Being able to see patient anatomy in full 3D visualization with contact force allows us to identify targets and navigate more efficiently,” said Gregory Michaud, MD, clinical director at Massachusetts General Hospital. “We look forward to continuing our use of the system as new features are developed."
In 2021, the company’s augmented reality tech received peer-reviewed analysis of its emergent applications. SentiAR says full clinical case studies are set to be published later this year.