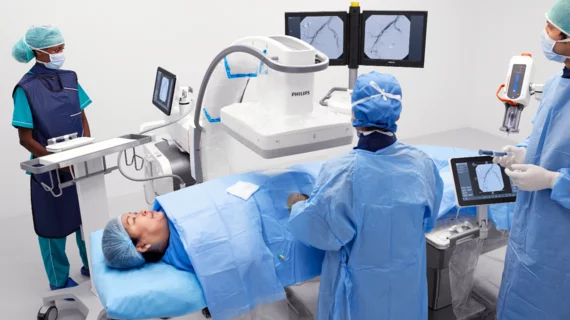
Philips’s new motorized C-arm earns FDA clearance
Philips has received 510(k) clearance from the U.S. Food and Drug Administration for its new motorized C-arm, the company announced this week.
The Zenition 90 Motorized was developed to meet the complex imaging needs of vascular procedures but can be used for a variety of different imaging applications in cardiology, pain management, urology and more. The motorized update to the C-arm removes the need for manual positioning adjustments during procedures, improving both accuracy and efficiency, a release on the equipment’s clearance states.
“During complex procedures, it’s vital to be able to rely on surgical imaging systems. As clinicians navigate their way through challenging anatomy, the priority is to quickly visualize small anatomical details while limiting X-ray dose,” Mark Stoffels, business leader for Philips Image Guided Therapy Systems, said in the release. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.”
The C-arm is equipped with a collision prevention system that detects the patient’s body within a certain distance and prevents unintentional contact that could break the sterile barrier, according to Philips product materials. In small independent studies conducted in the U.S. and Europe, 100% of providers who used the system said its table side operator component enabled complete control over the C-arm’s movements during procedures, Philips says.
Philips will be displaying the new C-arm during the 2024 Society for Vascular Surgery Annual Meeting taking place June 19-22 in Chicago.
To learn more, click here.