Large VA trial could change how patients are screened for liver cancer
A new study being conducted by the Department of Veterans Affairs could change the landscape of how patients are screened for liver cancer.
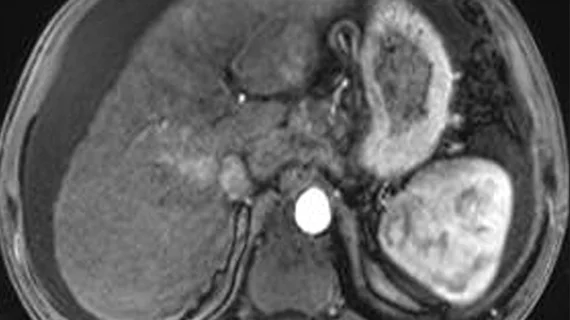
The study, which is said to be the largest of its kind, will use abbreviated MRI protocols to screen for liver cancer. MRI results will then be compared to those observed on ultrasound exams to measure each modality's performance. Experts involved in the work are hopeful that this new approach will lead to earlier cancer detection and better outcomes.
“VA has a high prevalence of patients with advanced liver disease who could benefit from screening for liver cancer. The study has the potential to change clinical practice for tens of thousands of Veterans and non-Veterans alike, and it could answer key questions about liver cancer screening that have been debated for more than three decades,” Assistant Under Secretary of Health for Discovery, Education and Affiliate Networks Carolyn M. Clancy, MD, said in a prepared statement about the upcoming research.
Ultrasound is typically a standard first step in the process of liver cancer screening. When lesions are detected via ultrasound, patients are then referred for MRI. But a challenge often encountered in ultrasound is that the quality of exams is patient and user dependent, which can result in lower sensitivity and missed or inaccurate findings. This could delay a liver cancer diagnosis.
Researchers involved in the new study propose that abbreviated MRI protocols, which take much less time to complete than traditional protocols, could offer more accurate assessments of liver lesions than ultrasound.
The trial, also known as PREMIUM (PREventing Liver Cancer Mortality through Imaging with Ultrasound vs. MRI), will recruit a total of 4,700 veterans starting this year and will take place over an eight-year period. Participants will undergo both ultrasound and abbreviated MRI of the liver to compare the modalities’ effects on outcomes, which has not been previously studied before, according to the VA’s release.
Co-chairs Dr. George Ioannou of the VA Puget Sound Healthcare System and Dr. Tamar Taddei of the VA Connecticut Healthcare System will lead the trial
For more information on the PREMIUM clinical trial, click here.