Battling Burns in MRI
While magnetic resonance imaging (MRI) is often considered one of the safer imaging modalities due to its lack of ionizing radiation, other dangers remain for patients, with thermal burns being a chief concern. The risk of burns is not a new concept, though we are still learning more about their causes and how to prevent them.
According to a Joint Commission Sentinel Event Alert which cited the Manufacture and User Facility Device Experience data base of the FDA, 70 percent of MRI complications are related to thermal burns.
“The single most common adverse event in the MR environment reported to the FDA is that of MRI burns,” says Emmanuel Kanal, MD, FACR, FISMRM, AANG, of the University of Pittsburgh Medical Center in Pennsylvania.
The primary cause of radiofrequency (RF) burns in patients undergoing MRI who are not wearing conductive devices is excessive power deposition, explains Devashish Shrivastava, PhD, of the University of Minnesota in Minneapolis. During MRI, RF power is not deposited uniformly into the patient’s body. Blood flow then redistributes this energy, resulting in non-uniform in vivo temperatures. Because certain in vivo regions become hotter than the rest, temperatures go beyond a safe value and RF burns occur.
Efforts have been made by regulatory bodies to determine safe temperature thresholds to prevent RF burns and other thermogenic adverse effects during MRI. “However, since in vivo temperatures could not be [and still cannot be] determined non-invasively with sufficient accuracy and precision suitable to ensure RF safety, equivalent safe numbers were computed using simple thermal transport models,” explains Shrivastava.
An issue looms large here: the models were never validated for MRI application and the inherent assumptions in their development resulted in overestimated power numbers. Moreover, the safe power numbers were not calculated for patients with altered blood flow and thermoregulatory characteristics. This includes patients with cerebral stroke, traumatic brain injuries and pregnancies.
“Patients with altered blood flow may have regions of tissue from where normal removal and transport of deposited power is compromised, making these patients more susceptible to MRI-induced thermogenic hazards,” says Shrivastava.
Because the safe power numbers are overestimated, hot regions are often times created deep inside the patient’s body like a microwave oven, which makes it harder for the patient to feel and for the technologists and clinicians to detect before it’s too late.
Utilizing healthy, human-sized, anesthetized pigs, Shrivastava and colleagues conducted a study to evaluate MRI- induced in vivo heating in a clinical 3T scanner. Pigs were used because they are thermo-physiologically similar to humans. Results demonstrated that safe temperature thresholds were violated within 10 to 15 minutes of RF power deposition. International regulatory guidelines allow a person to be scanned at full power continuously for an hour.
To best treat and prevent RF burns, Shrivastava urges technologists, clinicians and physicists to be very aware of the cues presented by their patients during MRI. If patients are overly sweating or complaining of being hot, they should be kept hydrated, ventilated and should be run at lower power values with frequent stops. “We can no longer assume that if the machine can be run at a higher power number it is safe,” he says.
He also suggests regulatory bodies recalculate safe power thresholds to make MRI safer. A three-pronged approach to making MRI safer is proposed by Shrivastava: relevant animal and human experimentations should be performed to generate data and understanding of the thermogenic hazards for different tissue types and physiology; first-principles-based, validated thermal transport models (e.g., generic bioheat transfer model) should be developed to fundamentally understand the thresholds of thermogenic hazards and to accurately compute in vivo temperatures to design safe imaging protocols; and accurate and precise MR-based in vivo temperature measurement techniques should be created to ensure that unacceptable in vivo heating does not occur.
“Addressing MR-induced thermogenic hazards will only make this modality safer and more useful for everyone,” says Shrivastava. “I urge everyone involved—manufacturers, clinicians, researchers—to be enthusiastically supportive of such efforts instead of shying away in fear of bad publicity. I sincerely believe this will be the right thing to do for the American people and the rest of the world.”
To ensure MR safety at each MRI site, a new international process is initiating a standardized personnel organization and structure. “You as the radiologist for a patient or the medical director of the MRI site will bear the responsibility not only for the accurate interpretation of the MRI examination to be performed, but also for its safe execution,” explains Kanal. “Whether or not we are the ones who hire or employ the technologists is entirely irrelevant to this direct line of responsibility that we owe to the patient. If you are interpreting MR examinations, it pays to become formally educated in the ethical and medicolegal responsibilities that de facto are yours for the safe execution of the MRI study, despite this coming as a surprise to so many of us in the field,” he says.
The burning truth about metallic microfiber in MRI
Though the latest athletic clothing may help you stay cool and look sharp while exercising in the gym, the same might not necessarily be said once you’re inside an MRI scanner. A report published in the May 2013 issue of the American Journal of Neuroradiology found that invisible metallic microfiber, which is found in popular athletic and “tech” clothing, presents an unrecognized MRI risk for cutaneous burn.
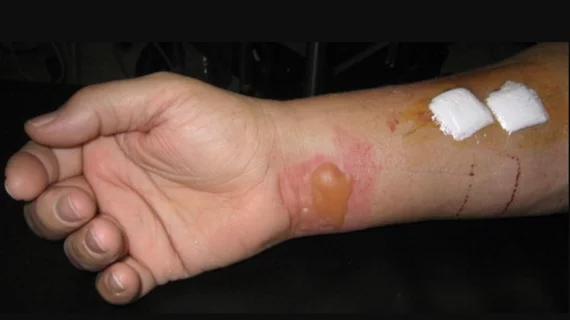
The article, whose lead author was Jeffrey M. Rogg, MD, of Rhode Island Hospital in Providence, reports a case of a second-degree burn sustained by an 11 year-old girl who presented for outpatient MRI of the spine for a scoliosis evaluation. The pediatric patient was wearing a gray undershirt under a long-sleeved white t-shirt and gray sweat pants. Prior to entering the scanner room, she was screened verbally, visually and with a wand metal detector. All appropriate protocols were followed during scanning.
Once the patient awoke from sedation, she complained of a burning discomfort on her right side. It was discovered that she had sustained linear erythematous blistering eruptions along her right flank and ipsilateral volar wrist where it contacted her undershirt.
“We retrospectively imaged the undershirt by high-resolution digital radiography and demonstrated an interleaving weblike pattern of radiopaque silver microfiber,” wrote Rogg and colleagues. The shirt had no visible label or evidence that it contained silver microfiber. The authors hypothesized electromagnetic eddy currents were generated within the shirt fiber that was concentrated at the fabric seam during the MRI, consequently leading to second degree skin burns along the sites of skin contact.
The authors urge vigilance to the prevention of thermal burns related to clothing as MRI systems with increasing field and gradient strengths become more ubiquitous. Rogg et al have revised their institution’s policy so that patients are required to change into site-supplied MRI-compatible outer clothing and wear nonmetallic cotton or other safe non-trade name undergarments for their exams. In exceptional circumstances, outpatients with severe physical constraints wearing clearly labeled 100 percent nonmetallic non-trade name fabrics are allowed to be scanned without changing.
The risk of burn is heightened even more so in sedated patients because they can’t vocalize any pain or discomfort. Greater scrutiny is needed with these patients.
“The common practice of not identifying the metallic components of multipurpose fabrics results in significant potential risk in the MR imaging environment,” wrote the researchers. “We demonstrate how, despite careful prescreening, invisible metallic microfiber in garments can fail to be detected before entering the MR imaging environment and potentially lead to patient thermal burns.”