New practice guidelines for PET imaging of neuroendocrine tumors
New practice guidelines for somatostatin receptor (SSTR) PET imaging relative to the diagnosis and treatment of neuroendocrine tumors (NETs) were released on Feb. 21.
Issued by the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM), the new guidelines are intended to inform any practitioners involved in ordering, performing, reading and/or reporting SSTR PET imaging.
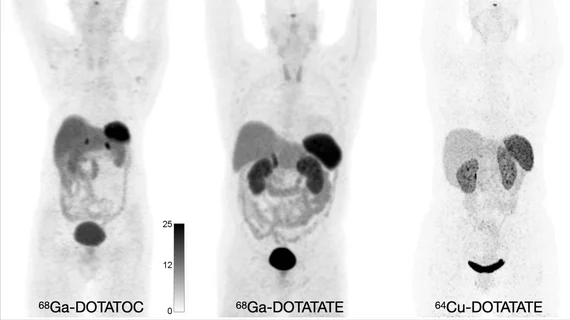
SSTR imaging using PET has recently overtaken scintigraphic imaging that targets somatostatin analogs (SSAs) using 111In-pentetreotide. Recent trends in SSTR PET imaging include the use of 68Ga-DOTATATE, 68Ga-DOTATOC or 64Cu-DOTATATE. These radiotracers come with several benefits, including increased lesion sensitivity, the need for less radiation and less time to complete scans.
The new standards address these recent changes and provide guidance on clinical indications, staff qualifications and requirements, exam specifications, reporting and documentation insight and information relative to dosing. They also contain data on quality control and quality assurance for the sake of accuracy and reproducibility in clinical trial environments.
Thomas A. Hope, vice chair of Clinical Operations and Strategy in the Department of Radiology and director of Molecular Therapy at the University of California San Francisco, commented on the development, noting that standardization of the procedure will help to ensure the exam’s appropriate use and promote its advancement in clinical trials.
“The SSTR PET procedure standard/practice guideline aims to provide clinicians with the best available evidence, to inform where robust evidence is lacking, and to help them to deliver the best possible diagnostic efficacy and study quality for their patients,” Hope said in a prepared statement.
The new guidelines are published in the Journal of Nuclear Medicine and can be found in full here.