Brain scans of patient who died while on experimental Alzheimer's drug cause experts to question safety
Some experts are questioning the safety of a new experimental drug that treats Alzheimer’s after a patient’s brain scans preceding her death revealed troubling findings.
Science reports that the 79-year-old Florida woman passed away after a series of severe headaches, confusion and seizures following an infusion of lecanemab—an experimental Alzheimer’s drug that targets amyloid and has been shown to significantly reduce functional and cognitive decline in individuals with early symptoms of the disease.
The woman was a participant in the drug’s core 18-month trial. It is not known whether she received the drug or a placebo during that time, but she did opt in for treatment in the extended phase of the trial, during which time she received at least two infusions of lecanemab.
According to reports, her family observed side effects following the first infusion. They escalated after the second infusion, when the patient began having seizures that resulted in hospitalization.
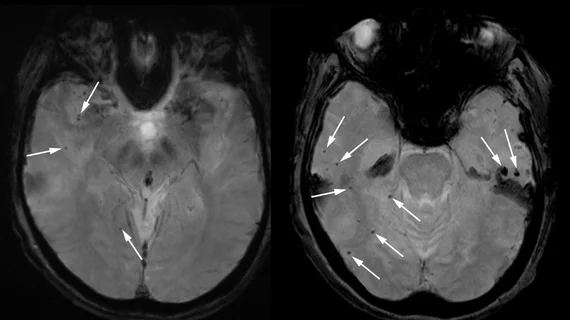
MRI scans of the woman’s brain revealed dozens of areas of bleeding and severe swelling. Boston University neurologist and neuroscientist Andreas Charidimou, who examined the patient’s records for Science, explained that the swelling was so severe in some cases that the folds of the cerebral cortex appeared “merged and squashed.” Charidimou described the findings as “a textbook case of severe ARIA.”
Increased incidence of ARIA—amyloid-related imaging abnormalities—were reported on the imaging of patients who received the drug during its 18-month trial. ARIA symptoms include headache, confusion, dizziness, nausea, etc., and were reported in just under 3% of trial participants who received the drug.
Charidimou and other physicians who were given access to the patient’s medical records and imaging elude that the brain scans indicate that the drug likely caused the woman’s brain swelling and bleeding and, ultimately, her death. This cannot yet be confirmed, but the woman’s family has arranged an autopsy to clarify her cause of death.
The drug’s initial trial included 1,800 participants. There were 13 deaths reported during that time, which experts concede are to be somewhat expected given the age and medical condition of participants. This latest death is the third that has occurred during the trial’s extended phase.