Imaging required to monitor effects of new Alzheimer's drug could inhibit its uptake
Although providers are optimistic about the potential of a new treatment for Alzheimer’s, some are concerned that the burden of monitoring the drug’s effect on patients could limit its uptake.
In January 2023, Eisai/Biogen's Leqembi—a drug shown to reduce clinical decline by up to 27% in patients with Alzheimer’s—was given accelerated approval by the U.S. Food and Drug Administration. It has been granted priority review and has a Prescription Drug User Fee Act (PDUFA) action date of July 6, 2023.
Spherix Global Insights recently conducted a new survey of 73 neurologists to gauge their intentions pertaining to prescribing the new drug. According to a published release on the survey data, neurologists are generally optimistic about the drug’s potential, with some already prescribing it. However, the reported side effects of the drug are not lost on the respondents.
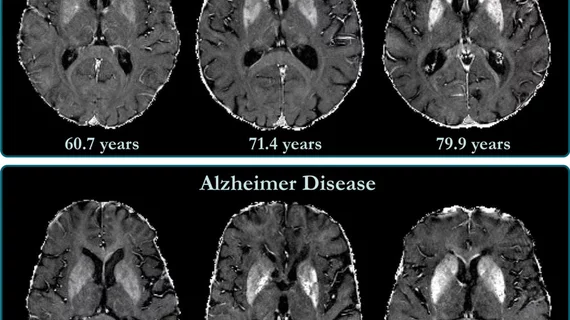
Leqembi has been shown to cause amyloid-related imaging abnormalities (ARIA). Symptoms of ARIA include headache, confusion, dizziness, vision changes and nausea.
ARIA is typically monitored via MRI exams (it can also be tracked on PET imaging). Patients being treated with Leqembi would require a series of scans in the months following the start of their treatment to assess both its effectiveness and any side effects.
In clinical trials, ARIA symptoms occurred in 2.8% of participants who received the drug. Despite the low incidence, ARIA remains a concern among many providers who would be prescribing the drug, according to the report.
One of the interviewed neurologists expressed apprehension around prescribing the therapy due to the monitoring burden for AIRA-related concerns, stating, “Every time a patient has a complaint, you're going to be saying, 'Do I need to scan them? Do I need the MRI? Is it ARIA?'”
That provider is not alone in their concerns. According to the release detailing the survey results, many respondents believe that ARIA-monitoring requirements could hinder uptake of the new therapy. Most expressed at least moderate concerns, while very few indicated that the benefits of the drug outweigh the risk of ARIA.
There are some blood-based tests available that could help ease the burden of monitoring patients being treated with the drug, but their use is not yet widespread.