New nuclear medicine treatment could potentially cure non-Hodgkin lymphoma
A new nuclear medicine therapy has the potential to treat and cure non-Hodgkin lymphoma, according to new research [1].
A study published in the April issue of the Journal of Nuclear Medicine details how the use of a single dose of [177Lu]Lu-ofatumumab eliminated tumor cells and significantly extended life in mice that had been injected with cancer cells.
To develop the therapy, researchers labelled human anti-CD20 antibody ofatumumab with the radioisotope 177Lu. They then established the in vitro characteristics of [177Lu]Lu-ofatumumab, estimated human dosimetry and tested its effectiveness in treating non-Hodgkin lymphoma in a mouse model.
Experts compared the treatment’s efficacy in mice that were either untreated, treated with unlabeled ofatumumab, treated with [177Lu]Lu-IgG or treated with varying doses of [177Lu]Lu-ofatumumab (0.74 MBq or 8.51 MBq).
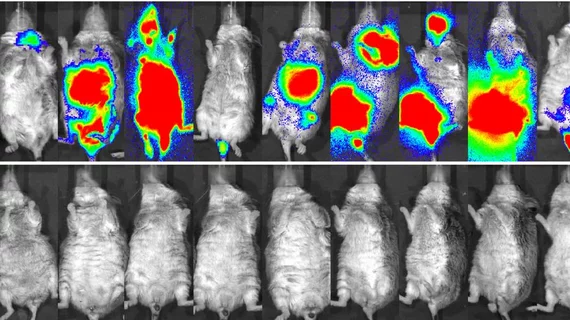
Mice that were not treated had a median survival of 19 days, whereas those treated with 8.51 MBq of [177Lu]Lu-ofatumumab had a median survival of 221 days—the best rate of all groups studied.
“What’s more, in mice treated with 8.51 MBq of [177Lu]Lu-ofatumumab, detectable tumors were eliminated completely within two days. Mice treated with the other therapies or left untreated, on the other hand, continued to show tumor cells present,” corresponding author of the paper Richard L. Wahl, MD, director of the Mallinckrodt Institute of Radiology at Washington University School of Medicine in St. Louis, and colleagues explained.
The authors added that they were able to produce the therapy with high yield and purity, and that its in vitro characteristics and dosimetry estimates indicate its potential for use in human subjects with non-Hodgkin lymphoma.
“The excellent therapeutic results in this animal model of human B cell lymphoma suggest that this curative treatment should be tested in humans with non-Hodgkin lymphoma,” Wahl suggested. “If testing is successful in humans, this would represent an excellent new treatment option for patients with this disease.”
The study can be accessed in full here.