Sterile barrier saves time and money for ultrasound-guided PIV insertions, new data show
Use of a new sterile barrier dressing for ultrasound-guided peripheral insertions could save organizations both money and time.
During the recent meeting of the Association for Vascular Access (AVA), experts shared a poster detailing the results of a single institution study involving a sterile dressing—UltraDrape—sharing that its use reduced associated insertion costs by 73% and insertion time by 50%.
“As the use of ultrasound guided peripheral access has increased with our difficult access patients, we have recognized the need to evaluate the procedural and supply variations from department to department,” the poster presented by study co-authors Nancy Moureau, RN, PhD, VA-BC, and Pam Pressnall, RN, VA-BC, reads.
Moureau and Pressnall cited concerns of varying aseptic techniques and waste when using standard central line dressing kits for ultrasound-guided peripheral catheter insertions. Both of these factors cost institutions time and money.
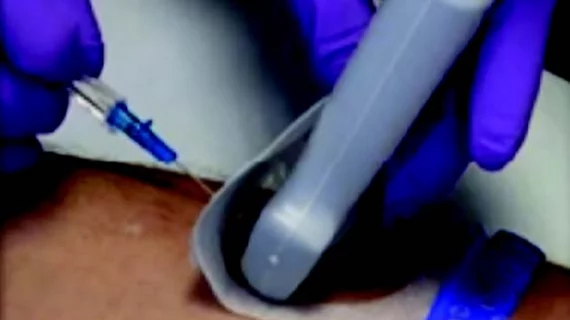
As part of a quality improvement initiative, the researchers switched to a standardized intravenous Start Kit with a sterile barrier ultrasound dressing for all PIV insertions. After the dressing is applied to the desired mark, ultrasound gel is applied to the back of it for probe guidance during the procedure. Once insertion is complete, the gel layer of the dressing is removed, and the rest is pulled down over the catheter to create a sterile dressing cover.
The experts shared that the switch in kits reduced the price per insertion from $25.32 to $6.71, while also decreasing procedure times from 6.51-12.14 minutes to 3.2-4.25 minutes. It was estimated that this would result in cost savings of more than $20,000 per year, according to the data provided.
“This helped us achieve a level of efficiency that allows us to perform more ultrasound-guided patient insertions in fewer attempts, resulting in happier patients and nursing staff,” Pressnall said in a release.
Disclosure:
UltraDrape is a product of Parker Labs. Parker Labs provided in-kind product, education and editorial support for the study, but did not fund it.