New wireless sensors monitor sick, premature babies without disrupting imaging exams
Researchers from Northwestern University in Evanston, Illinois have developed a new way to monitor sick and premature newborns in hospitals’ neonatal intensive care units (NICU) using skin-like, battery free, wireless sensors that are completely compatible with medical imaging technology.
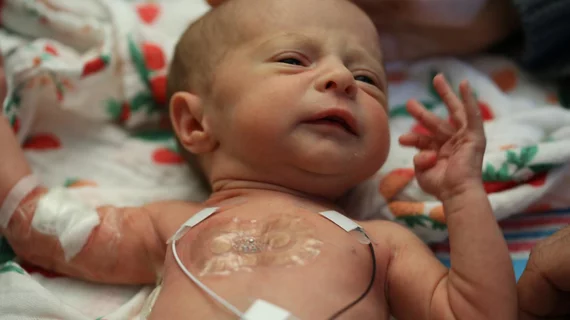
The sensors, detailed in a study published online March 1 in Science, provide data as accurate as that from traditional, and often wire-heavy monitoring systems. They also have adhesives that are gentler on a newborn’s skin and allow for more physical bonding between the baby and parent.
"We wanted to eliminate the rat's nest of wires and aggressive adhesives associated with existing hardware systems and replace them with something safer, more patient-centric and more compatible with parent-child interaction," lead author John A. Rogers, PhD, a professor of biomedical engineering and neurological surgery at Northwestern, said in a prepared statement from the university. “Our wireless, battery-free, skin-like devices give up nothing in terms of range of measurement, accuracy and precision—and they even provide advanced measurements that are clinically important but not commonly collected."
For their study, Rogers and colleagues put the sensors on roughly 70 premature babies at Northwestern-affiliated NICUs.
Current technology uses electrodes with strong adhesives on the baby that are connected to large computing systems and surround the baby’s incubator. The adhesives can also damage and potentially scar the developing skin of the babies.
In contrast, the dual wireless sensors involve one sensor that sticks to the baby’s chest or back while the other wraps around the foot. This allow physicians to determine an infant’s core temperature and body temperature from a peripheral region and further determine blood flow, blood pressure, respiratory rate and cardiac function, according to the researchers. The devices are cheap enough to be discarded after 24 hours and replaced with new ones to reduce risk of infection.
The sensors, powered by radiofrequency energy, collect vital signs data and use Bluetooth technology to transmit the data to mobile devices acquired by nurses and staff.
The devices are also largely radiolucent and can be worn during x-rays, MRIs and CT scans so imaging can be conducted while monitoring of the baby continues.
“MRI and x-ray imaging are possible without removing the devices and they are also optically transparent, to a large extent,” Rogers told Health Imaging. “The conventional hardwired sensors are not optically transparent, and they are also not radiolucent—they must be removed for x-ray or MRI.”
The device could also help physicians measure the infants’ vital signs while being held by their parents without the obstruction of wires, which may give more insight into the baby’s health.
Rogers predicts his team's sensors will appear in U.S. hospitals in two to three years. The researchers also hope to receive FDA approval in the next five years, according to an article by Axios, and expect to send sensors to developing countries over the next year.