How CMS Grants New Technology Add-On Payments and Determines Substantial Similarity
New Technology Add-On Payments (NTAP) are a class of reimbursement that are meant to help pay for new technology that is not included in the DRG bundled payment. Specifically, NTAP recognizes that current DRG payment rates can be a barrier to adopting new technology and represents an additional payment for hospital stays that use new technology determined by CMS to provide substantial clinical improvement and where the current DRG payment would be inadequate. NTAP applications are evaluated as part of the annual Inpatient Prospective Payment System (IPPS) rulemaking process. If an NTAP is approved it remains in effect for 3 years following approval, at the end of which it is assumed that the costs of the new technology have been absorbed into the core DRG through the yearly DRG update and calibration process.

MPP, President & CEO
Healthcare Cost Institute
CMS’s decision to grant the first NTAP for AI-enabled stroke intervention and care was a critical regulatory milestone in the development and adoption of AI-enabled health care. As part of the application, Viz.ai had to demonstrate that it met the newness, cost and clinical improvement criteria. In the final rule CMS determined that Viz.ai had demonstrated that their product ContaCT reduced time to treatment, has high specialist engagement and improved patient outcomes [CMS, PDF]
Since the NTAP approval, other start-up companies have claimed the new reimbursement applies to their software solution. To date more than five companies have claimed the rights to the code, ranging from stroke triage, to radiology prioritization, and even to CAD. The question is, does this code apply to everyone who wants it? I did some deeper digging to clarify.
The role of substantial similarity
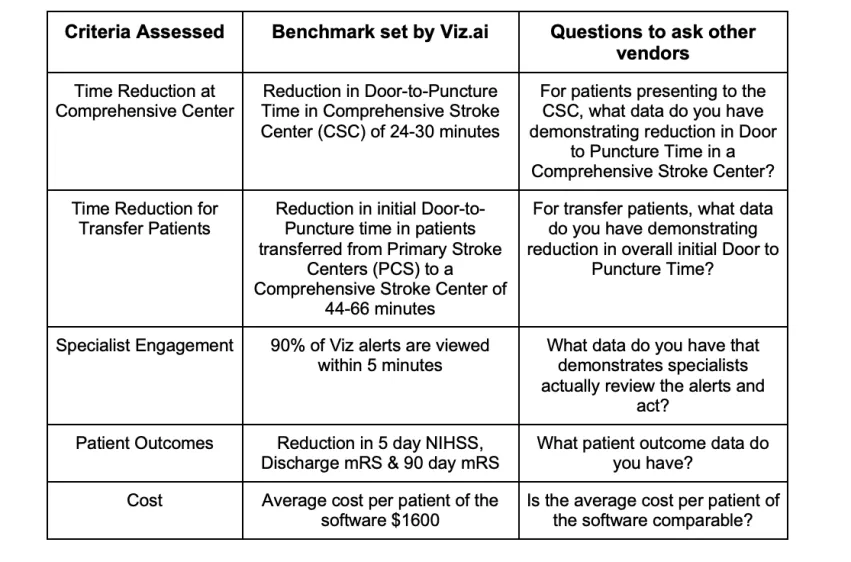
To date no other AI technologies have been approved by CMS. CMS has however stated that the intent of NTAP is not to provide an advantage or reward to a single vendor therefore if other vendors provide evidence of substantial similarity, their technology should also be able to achieve NTAP. In order to demonstrate substantial similarity, a company must demonstrate its technology meets all relevant criteria, which in this case includes substantial clinical improvement, time to treatment, specialist engagement, patient outcomes, newness and cost (outlined in the table below). Traditionally, a substantial similarity determination has been applied via new NTAP applications, and CMS has determined whether products are substantially similar and have told applicants whether they are eligible for coverage under the initial NTAP. Where things start to get problematic is if, instead of CMS determining substantial similarity, other vendors unilaterally assert substantial similarity in order to “piggyback” on an existing NTAP. Without official word from CMS hospitals have to choose whether to take a risk - as the decision lies with individual hospitals' risk tolerance as to the technology they want to use and submit for NTAP payments. CMS requires all hospitals to adhere to “coding hygiene” standards in order to ensure that the correct codes are being submitted for the correct services. It's also possible that the American Hospital Association may provide some coding clarity regarding what specific technologies qualify for NTAP payments. Hospitals considering which technology to use should consider asking the following questions: