FDA clears AI software capable of reducing MRI acquisition times by up to 80%
A new artificial intelligence-based software aimed at improving the quality of MR imaging was just cleared by the U.S. Food and Drug Administration.
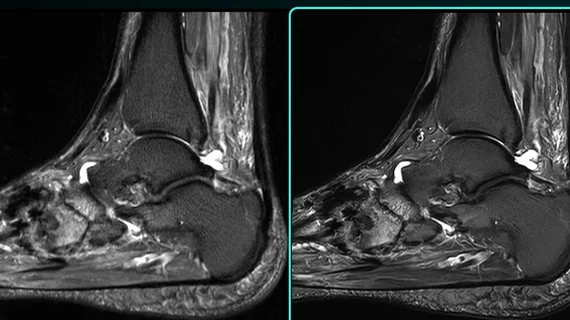
Subtle Medical—a company that specializes in AI-powered medical image enhancement—announced the clearance of its Subtle-HD software on Wednesday. The product was developed to both improve image quality and shorten image acquisition times. It provides advanced denoising and image sharpening techniques and is said to reduce scan times by up to 80% for some protocols.
With the clearance of Subtle HD also comes the launch the Subtle Elite platform, which integrates three of the company’s AI solutions—SubtleHD, SubtleSYNTH and SubtleALIGN (all FDA cleared). Combined, the products can significantly improve patient comfort and radiology workflows by enabling techs to acquire high quality imaging with reduced variability in substantially less time.
The suite is vendor neutral and can be integrated into all MRI scanners, regardless of manufacturer or model.
In an announcement on the clearance, Chief Product Officer Ajit Shankaranarayanan, PhD, highlighted the potential the suite has for increasing patient throughput and revenue without the need for additional equipment or infrastructure upgrades.
"The FDA clearance of Subtle-HD marks a significant milestone in AI-driven radiology, giving hospitals and imaging centers a powerful solution to increase efficiency, improve image quality, and optimize workflows—all without additional hardware costs," Shankaranarayanan said. "By combining SubtleHD, SubtleSYNTH, and SubtleALIGN into a single, comprehensive package, Subtle-ELITE™ is setting a new benchmark for the future of MRI."
Subtle Medical was founded in 2017 by Greg Zaharchuk, MD, PhD, a neuroradiologist and Professor at Stanford, and Enhao Gong, PhD, a deep learning scientist. Together, the two have significantly expanded the company’s growth. They now have more than 70 full-time employees, 8 FDA clearances and over 30 issued or pending patents. Their AI solutions have been integrated into over 1,000 scanners across dozens of countries.
Learn more about their latest clearance here.