GE Healthcare doubling distribution of imaging tracer to meet expected demand for new Alzheimer’s drug
GE Healthcare is ramping up its distribution of Vizamyl F18-PET imaging tracers to meet expected demand created by the federal approval of Biogen’s groundbreaking Alzheimer’s treatment.
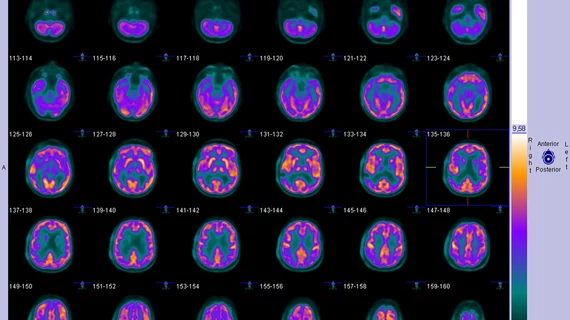
Flutemetamol F18 is a radiotracer used to diagnose beta-amyloid plaques in patients with suspected Alzheimer’s disease or other cognitive disorders. Individuals seeking treatment with Aduhelm (aducanumab) must have either an amyloid PET scan or lumbar puncture showing amyloid presence in the brain. They also must undergo brain MRI scans at mandated time intervals during treatment to track progression.
Taken together, providers and industry groups envision a growing demand for imaging and radiotracers used to track amyloid plaques.
“For years we’ve been talking about precision health and theranostics as ‘the future of healthcare.’ Now, we are seeing it become a reality,” Jean Luc Procaccini, president & CEO of Molecular Imaging & Computed Tomography at GE Healthcare said in a statement.
On Wednesday, a 70-year-old Rhode Island resident became the first person treated with the controversial drug, Reuters reported June 16. Biogen estimates up to 1.5 million of the 6 million people in the U.S. with Alzheimer’s will be eligible for Aduhelm therapy.
Packing inserts on the drug don’t currently mandate amyloid PET scans to initiate treatment, but the Society of Nuclear Medicine and Molecular Imaging says it will be working to reverse that policy.
GE Healthcare’s move is part of a larger effort to expand molecular imaging products and solutions announced during the Society of Nuclear Medicine and Molecular Imaging’s annual meeting last week.