Breast density notification laws: FDA provides updated timeline on rollout
The U.S. Food and Drug Administration (FDA) has shared when they anticipate the long-awaited breast density notification rule to roll out.
In response to a letter from U.S. Congresswoman Rosa DeLauro, who recently requested that the FDA share an update on the proposed breast density notification regulation changes, the agency shared that it anticipates the final rule amending portions of the Mammography Quality Standards Act of 1992 (MQSA) will publish before the end of 2022 or by the beginning of 2023. The new rules were originally proposed and gathered public comment in 2019 just prior to the pandemic.
“FDA remains committed to advancing our MQSA rulemaking and to ensuring that patients can access safer, more reliable mammography services, and that providers are supported by the current science in order to improve outcomes and reduce suffering,” the FDA letter reads.
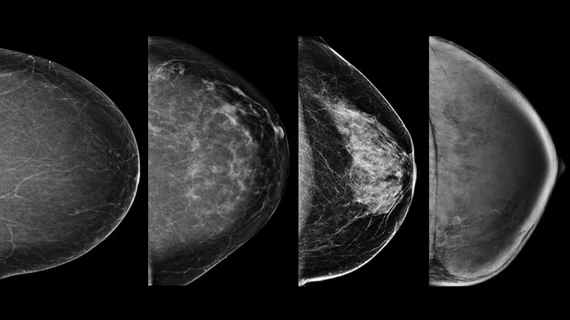
Although many states (38) have some level of breast density notification laws in place, not every state requires providers to notify patients of their breast density status. In fact, some states do not even require that an effort to inform patients of their individual breast density status be made by providers at all. The proposed regulations aim to change that by requiring all healthcare providers to offer patients a summary of their breast density (whether it is low or high) that details their breast cancer risks and covers any additional screening options that may be available.
“I was proud to help enact the Breast Density and Mammography Reporting Act, which required FDA to develop this policy,” Rep. DeLauro, Chair of the House Appropriations Committee, said in a statement about the FDA update. “Let me be clear: women’s lives are at stake. I urge the FDA to move swiftly to release the final rule before the end of the year.”
This bill amends the Public Health Service Act to require mammography facilities to include up-to-date information about breast density in both the written report of the results of a mammography examination provided to a patient's physician and the summary of that written report given to the patient. The summary must convey: (1) the effect of breast density in masking the presence of breast cancer on a mammogram, and (2) that individuals with dense breasts should talk with their healthcare providers about any questions or concerns regarding the summary and whether they would benefit from additional tests, according to a summary posted on densebreast-info.org.
The bill requires the Department of Health and Human Services to expand and intensify research on breast density, the cost-effectiveness and feasibility of supplemental imaging relating to breast density, and best practices concerning mammograms and supplemental screening for those with dense breasts.
The proposed regulations could also open the door to establishing a national reporting standard pertaining to breast density notifications—something that JoAnn Pushkin, executive director of DenseBreast-info.org, and a breast cancer survivor herself, has long advocated for.
“The 38 states with existing inform laws provide varying levels of notification. A single national reporting standard would mean all American women receive equal information about the screening and risk implications of dense tissue and provide equal opportunity to discuss supplemental screening leading to earlier detection,” Pushkin said in a statement applauding the push from Rep. DeLauro.
For more information on the proposed regulations, or to read the full FDA response to Rep. DeLauro’s request for an updated timeline, click here.