Researchers identify new PET imaging biomarker capable of predicting immunotherapy success
A newly identified PET imaging biomarker could help providers tailor immunotherapy treatments for patients with certain types of cancers.
Research in the Journal of Nuclear Medicine reveals the protein galectin-1 (Gal-1), of the carbohydrate-binding lectin family, as a new imaging biomarker for immune checkpoint blockade (ICB) therapy. Authors of the study suggest that the biomarker could be used to identify people most likely to benefit from this particular treatment.
“Developing reliable approaches for assessing responses and selecting eligible patients for immunotherapy remains challenging,” Zhaofei Liu, PhD, Boya Distinguished Professor at Peking University in Beijing, China, noted. “Current clinical criteria for monitoring solid tumor responses to immunotherapy are based on CT and MRI scans, but these methods result in a considerable delay between treatment commencement and response evaluation.”
Molecular imaging offers providers more quantitative data in real-time to base disease management decisions on, and using PET biomarkers has the potential to improve outcomes via targeted therapeutics, Liu added.
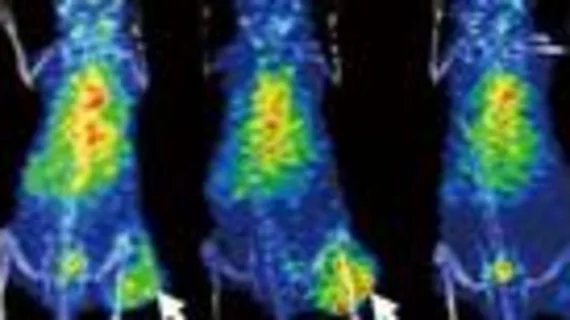
In the study, researchers conducted proteomic analysis on mice to determine whether the expression of certain proteins had any effect on responses to ICB therapy. Through this, they found that tumors with low expression of Gal-1 responded favorably to the treatment.
The team then labeled the protein with 124I and the radiotracer (124I-α-Gal-1) before conducting PET scans on the subjects prior to starting ICB therapy. This highlighted the pretreatment immunosuppressive status of the tumors’ microenvironment, informing researchers on the potential for ICB resistance.
The finding “opens avenues for the early prediction of ICB efficacy before treatment initiation and facilitates the precision design of combinational regimes,” the group suggested, adding that it could enable precision treatment in the future once confirmed in additional research.
The full study can be viewed here.