New algorithm is twice as accurate at predicting stroke timing compared to the standard of care
A new artificial intelligence algorithm could be the key to improving estimations of stroke timing based on CT imaging.
In fact, a recent analysis of the algorithm’s performance revealed that it is twice as accurate as standard visual assessments of CT exams for determining the onset of stroke. Its performance was detailed recently in NPJ Digital Medicine, where experts described their use of machine learning techniques to develop an algorithm that they believe has the potential to improve patient outcomes.
“Advantages of ML are that it screens high-dimensional imaging features for associations with ischemia progression, including those imperceptible to experts, and can account for lesion anatomy variability and signal heterogeneity,” study leader Paul Bentley, PhD, with the Department of Brain Sciences at Imperial College London, and colleagues explained. “ML has proven superior to unidimensional approaches for MRI-based ischemic lesion age estimation, and for [noncontrast CT]-based prediction of edema. ML also enables objective lesion segmentation from NCCT images, that can secondarily be analyzed for lesion age.”
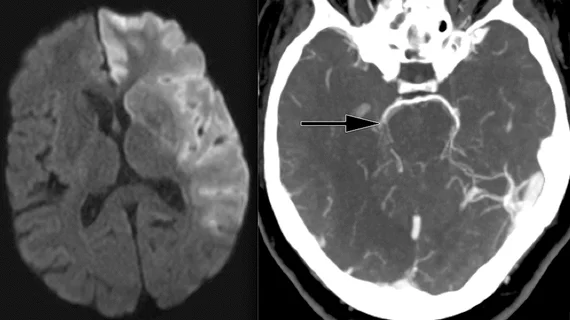
Determining the age of brain lesions is critical for stroke management, as there is a small window of time for initiating treatment that can inhibit damage. However, it can be challenging to establish when a stroke starts, especially if there are communication barriers or patients are not accompanied by another person when they present with symptoms.
A team of experts from Imperial College London, the University of Edinburgh, and the Technical University of Munich (TUM) collaborated to develop an algorithm that can estimate lesion age using standard noncontrast CT images. Researchers trained a convolutional neural network-radiomics (CNN-R) model to predict chronometric lesion age using a dataset of more than 800 CT scans with known stroke times.
When tested on a subset of 2,000 scans, the model proved itself to be twice as accurate as standard methods of visual estimation. It also performed well at estimating the “biological age” of damage identified on CT scans, offering providers additional insight into the extent of the stroke damage and helping to rule out treatment methods that may not be feasible based on how much time has lapsed.
Leibniz Prize winner Daniel Rückert, Professor of Artificial Intelligence in Healthcare and Medicine at TUM, who also took part in the research, commented on the model’s potential in a release on the findings.
"We believe that our model is so powerful because it not only assesses how dark the damaged region is, but also includes additional features from the scans, such as texture, and accounts for variations within the damaged areas and background,” Rückert explained.
The team is optimistic about their model’s potential to improve outcomes and suggested that it could be utilized in up to 50% of stroke cases in the future.
“Having this information at their fingertips will help doctors to make emergency decisions about what treatments should be undertaken in stroke patients,” Bentley said. “Not only is our software twice as accurate at time-reading as current best practice, but it can be fully automated once a stroke becomes visible on a scan.”
The study abstract is available here.