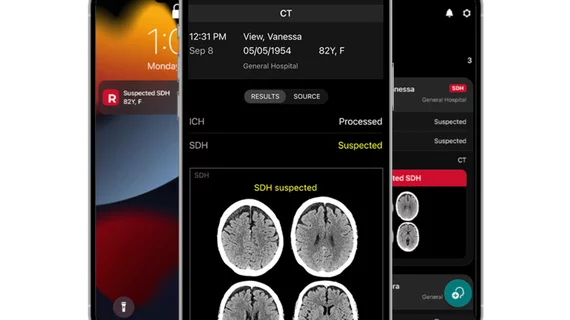
RapidAI receives FDA clearance for subdural hematoma alert module
RapidAI, a developer of artificial intelligence (AI) for critical medical conditions, announced it has received FDA clearance for its Rapid SDH, an AI module for automatically detecting hemispheric subdural hematomas.
The tech notifies trauma teams across the health system automatically when a patient is suspected of having a hemispheric subdural hematoma or major brain hemorrhage, facilitating rapid intervention of what could be a serious critical emergency.
RapidAI says the alerts are sent out in under a minute after the AI analyzes scans.
"The FDA's clearance of Rapid SDH significantly enhances our expanding range of hemorrhagic and trauma care solutions at this crucial time of rapidly growing patient numbers, clinician shortages, and advancements in potential treatment options,” Amit Phadnis, CTO of RapidAI said in a statement. “Our goal is to continue to expand the capabilities and applications of our deep AI to deliver comprehensive clinical solutions that provide care teams with the crucial insights necessary to evaluate patients, streamline decision making, and expedite care for this common and dangerous disease.”
According to the company, Rapid SDH offers a positive predictive value (PPV) of 99% for suspected acute and chronic subdural hematomas. Clinical alerts are sent through the Rapid mobile app, email, and can also be integrated into hospital image archiving and communication systems.
RapidSDH integrates into other modules that detect a variety of emergency conditions that affect the brain, including aneurysm and stroke.
Development comes after research data showed a projected 80% increase in subdural hematoma cases in US patients by 2040, coupled with existing high mortality rates and shortages in the neurosurgical workforce. Currently, mortality rates are 40 to 60%.