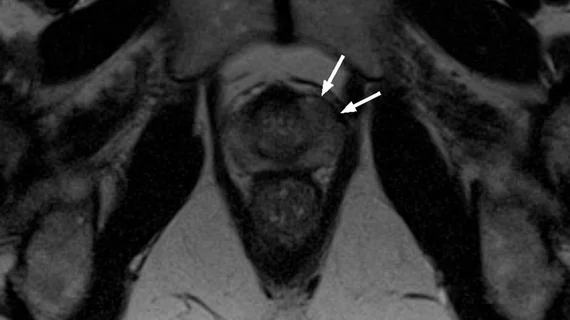
Routine imaging is a suitable alternative to one-year confirmatory biopsy during active surveillance of prostate cancer, new analysis shows.
This was the conclusion of a paper published recently in the Journal of Urology. In the paper, researchers detailed the oncologic outcomes of 172 men with prostate cancer when yearly multiparametric MRI exams were completed in lieu of biopsies during the first and second year following the patients’ diagnosis. The results of their work indicated that risks are not exacerbated when imaging is the chosen method of surveillance up until the year three mark [1], suggesting patients can safely forego invasive biopsy for a limited period.
Patients involved in the study initially underwent baseline MRI scans in addition to targeted biopsy. After that, multiparametric MRI exams were completed for each patient annually for two years following their diagnosis.
Through this, the experts found that both MR imaging and prostate specific antigen density were significant predictors of disease progression. Just 4 out of 72 patients had false-negative imaging and high-risk pathological features. At a median follow-up of 69 months the majority of patients were free of biochemical recurrence, metastasis and prostate cancer–related death (99.3%, 100% and 100%, respectively).
“Final analysis of the Magnetic Resonance Imaging in Active Surveillance trial indicates that there is minimal risk to omitting 1-year confirmatory biopsy during active surveillance if baseline magnetic resonance–targeted + saturation template biopsy was performed,” corresponding author Paul Doan, of the Garvan Institute of Medical Research and The Kinghorn Cancer Centre in Australia, and colleagues concluded.
The researchers did, however, recommend targeted biopsy at the three-year mark due to the risk of “occasional magnetic resonance imaging–invisible tumors.”
The study abstract can be viewed here.