Philips wins FDA clearance for new suite of ultrasound tools measuring liver health
Philips has gained federal clearance for its new suite of ultrasound tools developed to enhance treatment for those with early-stage liver diseases.
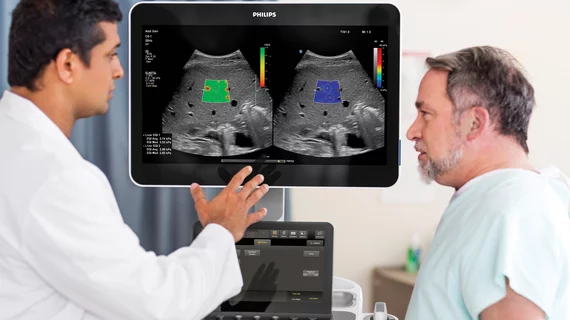
The Food and Drug Administration announced the 510(k) clearance for Philips’ liver fat quantification line on Tuesday. Radiologists armed with IPIQ Elite and Affiniti products can now more easily assess liver disease progression while reducing costs and increasing patient access to sonography.
“Accessible ultrasound-based liver fat quantification is a screening and early diagnostic tool that will allow many more patients to take their health into their own hands by making simple lifestyle changes,” General Manager of Ultrasound at Philips Jeff Cohen said in a statement.
Fatty liver disease remains the most common form of chronic liver disease and spotting signs of the condition early is crucial to possibly reversing disease progression.
The new clearance will also help technologists access guidance and decision support mechanisms in real-time, enhancing diagnostic certainty and streamlining workflows, according to Philips.
“With traditional gray scale imaging, we could only tell if the liver had a high degree of fatty infiltration or if it was normal, but it was very hard to assess whether fatty liver disease was mild, moderate or severe,” Richard G. Barr, MD, PhD, president of Radiology Consultant Inc., and Medical Director at Southwoods Imaging in Boardman, Ohio. “Attenuation imaging now gives us a numerical value that will enable us to follow the patient over time.”
Philips will feature its new liver health tools during the RSNA annual meeting, taking place Nov. 23-Dec. 2 in Chicago.