FDA clears redesigned mammography platform from Siemens Healthineers
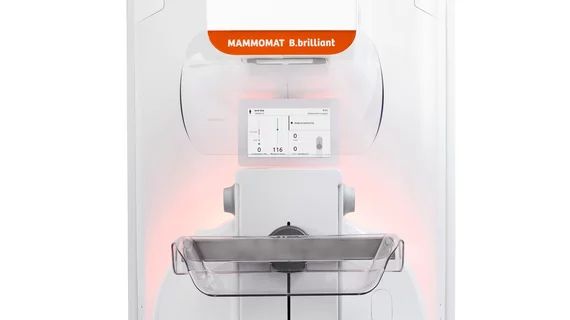
Siemens Healthineers has obtained U.S. Food and Drug Administration clearance for its Mammomat B.brilliant, a complete rebuild of its mammography platform, designed to improve patient comfort. This marks the first major redesign from Siemens in over a decade.
Patient comfort is prioritized through features like the ComfortPackage, including ergonomic hand grips, optimized face shields, ambient light displays, and personalized breast compression paddles. For technologists, enhancements such as a prominent display monitor, automated ComfortMove ergonomic feature and laser positioning guide aim to reduce physical strain.
“We believe more women deserve access to next-generation screening technology, and this innovation underlies our commitment to women’s health,” Niral Patel, vice president of X-ray Products at Siemens Healthineers North America said in a statement sent to Health Imaging.
Submitted for additional clearance is a 3D tomosynthesis technology that promises improved depth resolution and tissue separation through innovative detector and X-ray tube designs, as well as UltraHD reconstruction technology for enhanced imaging capabilities. Once cleared, it will be part of the Mammomat B.brilliant platform.