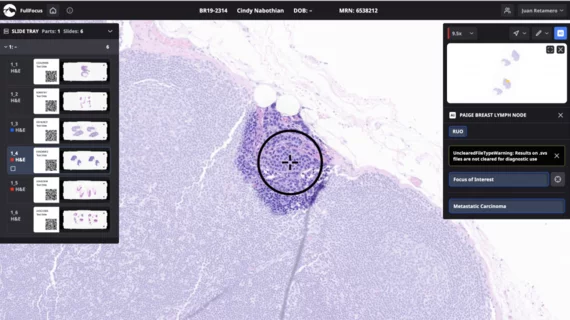
FDA grants Paige ‘Breakthrough’ status for breast cancer detection
The U.S. Food and Drug Administration (FDA) has granted Paige, a technology company that supports cancer diagnosis, Breakthrough Device Designation for its artificial intelligence application, Paige Lymph Node. The Breakthrough Device Designation is awarded to technologies with the potential to improve diagnosis or treatment of life-threatening diseases.
Paige Lymph Node is designed to assist pathologists in detecting breast cancer metastases in lymph node tissue. Its AI model utilizes deep learning trained on over 32,000 digitized lymph node slides. The software highlights areas of concern, aiding pathologists in their assessments.
According to a statement about the Breakthrough status, Paige Lymph Node shows near-perfect sensitivity in detecting breast cancer metastases.
“Pathologic assessment of lymph nodes in breast cancer patients is critically important for prediction of outcome and treatment, yet the process is time-consuming and error-prone,” David S. Klimstra, MD, founder and chief medical officer at Paige, said in the statement. “Paige Lymph Node uses the power of AI to help the pathologist identify even small lymph node metastases rapidly and accurately, ensuring that breast cancer patients receive the optimal management of their disease.”
Paige previously received Breakthrough Designation for Paige Prostate Detect, the first FDA-authorized digital pathology application. However, according to the statement, Paige Lymph Node is the first AI application of its kind to receive this designation from the FDA.