Lunit AI-powered 3D breast imaging receives FDA clearance
Lunit, a South Korean developer of artificial intelligence for cancer diagnostics, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 3D Breast Tomosynthesis (DBT) AI product, Lunit Insight DBT.
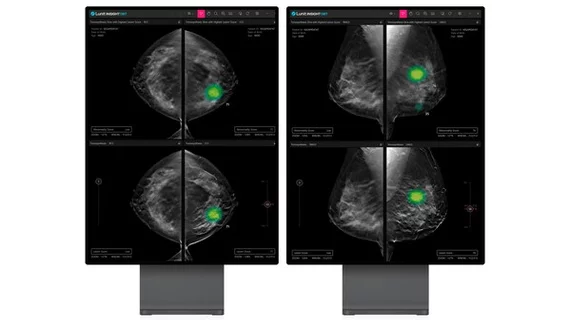
Insight analyzes 3D images from DBT equipment, offering a potentially faster and more accurate diagnosis compared to traditional 2D mammography screenings. The AI is a deep learning model that measures the likely malignancy of suspicious breast lesions, automatically providing details to assist providers, including level of lesion calcification and what type of tissue it is embedded in.
In its announcement, Lunit said Insight enables radiologists to zoom in and view the lesion in detail to help clinical teams make an informed decision on next steps for diagnosis and potential treatment, all in an effort to reduce unnecessary biopsies.
FDA clearance allows Lunit to deploy Insight in medical settings in the U.S., where it can be used for breast cancer examinations. According to Lunit, the United States accounts for over 64% of the global demand for 3D breast imaging.
"The U.S. is the biggest player in the global breast screening market, accounting for up to 40% of the market share. More than 40 million mammography screenings are reported in the U.S. annually," Brandon Suh, CEO of Lunit said in the statement. "Given this substantial market influence, achieving FDA clearance for Lunit Insight DBT not only solidifies our presence in the largest market but also marks a significant milestone in our mission to revolutionize breast cancer diagnosis and, ultimately, save more lives."
Lunit received clearance for the use of Insight in Europe in March.