Follow-up imaging for reactive adenopathy s/p COVID booster might not be necessary
Experts recently gained a better understanding of how axillary lymphadenopathy presents on breast MRI following COVID boosters.
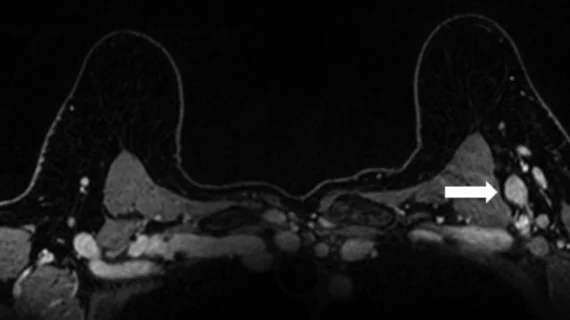
Reactive adenopathy is a well-documented side effect of COVID vaccination, but less is known about how boosters affect lymph nodes on imaging. On breast MRI, swollen lymph nodes are most likely to present with an increase of cortical thickness of .2 cm or more, according to new data published in Academic Radiology.
“As more studies reveal the frequency of lymphadenopathy following initial COVID-19 vaccination, much about this association remains unknown,” corresponding author of the paper Joao V Horvat, MD, with the Department of Radiology at Memorial Sloan Kettering Cancer Center in New York, and colleagues explained. “There is a lack of information available in the literature regarding the frequency and appropriate management of lymphadenopathy in patients after the COVID-19 booster vaccination.”
The new research utilized breast MRI exams conducted on 128 women before receiving a COVID booster and after (median of 31 days in between booster shot and second MRI) to compare axillary lymph node morphology. Increased cortical thickness of .2 cm or greater was noted in 19% of the study’s participants, with those who had received their booster more recently being likelier to display the swelling. These patients also showed an increase in long and short axes.
None of the patients were subsequently diagnosed with breast cancer ipsilateral to their booster, and age and vaccine type (Moderna, Pfizer, etc.) were not found to affect the observed thickening on imaging.
This finding prompted the authors to suggest that follow-up imaging of axillary lymphadenopathy s/p COVID booster may be unnecessary in patients who have a low risk of malignancy.
“As more information was gathered in the literature, the perceived need for imaging follow-up diminished,” the authors wrote. “Given the high frequency of lymphadenopathy following COVID-19 vaccination, further evaluation or follow-up may not be necessary in patients without a suspicious finding within the breast.”
The study abstract is available here.