Generic, FDA approved contrast agent set to hit the market in wake of nationwide shortage
On July 18 Fresenius Kabi announced that they are rolling out a line of generic contrast media products, starting immediately with Iodixanol Injection USP.
Iodixanol Injection USP is bioequivalent and therapeutically equivalent to Visipaque, and can be used for a myriad of intra-arterial and intravenous diagnostic radiology procedures, including contrast-enhanced CT scans, urography, angiography and more.
The announcement comes on the heels of a nationwide contrast shortage that has impacted tens of thousands of imaging procedures since April. With some leaders in the imaging community hinting that the contrast shortage could linger into the fall, the availability of a new option should come as welcome news to patients and providers.
“Fresenius Kabi is pleased to help expand access to affordable, high-quality contrast media agents for the radiology community,” Fresenius Kabi USA president and CEO John Ducker said in a statement. “The approval and U.S. availability of Fresenius Kabi Iodixanol Injection USP is expected to provide immediate relief to the current shortage. As a company committed to the purpose of ‘caring for life,’ we’re honored to help patients receive the timely care they need.”
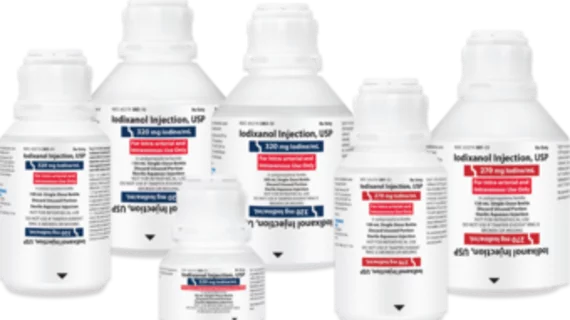
The agent has been approved by the U.S. Food and Drug Administration. It is preservative-free and comes in six different presentations for imaging procedures:
270 mg Iodine per mL:
100 mL polymer bottle
150 mL polymer bottle
320 mg Iodine per mL:
50 mL polymer bottle
100 mL polymer bottle
150 mL polymer bottle
200 mL polymer bottle
Lindsey Thomas, senior vice president of pharmaceutical marketing at Fresenius Kabi USA, shared that Iodixanol Injection USP is the first of several generic imaging agents the company is making available to consumers. She emphasized that the current focus is on expediting availability:
“We are doing everything we can to accelerate product availability to help our customers during this acute contrast media agent shortage, including air shipping product. We are also actively working to bring additional affordable contrast agents to U.S. clinicians to help ensure patient access to essential diagnostic imaging procedures.”
For more safety, adverse event, contraindication and prescribing information, click here.