FDA announces Class I recall of 150 MRI scanners due to risk of explosion
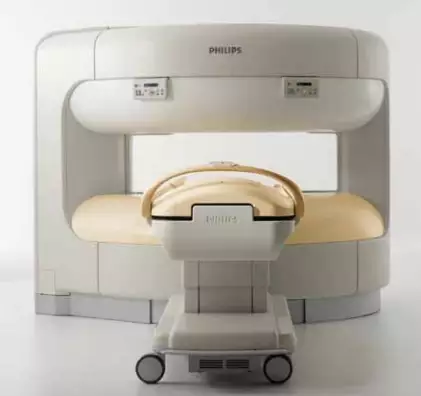
The U.S. Food and Drug Administration (FDA) has announced that Philips North America is recalling some of its Panorama 1.0T HFO MRI systems due to a risk of explosion. This is a Class I recall, which means using these devices "may cause serious injuries or death."
The recall is prompted by concerns that during a quench, an infrequent process involving the release of a large amount of evaporated helium through a venting system, an unknown blockage could compromise the venting system's integrity, potentially leading to chemical exposure, lack of oxygen, tissue damage or serious injury.
Although only one explosion has been reported in 22 years of use, the severity of potential incidents necessitates the recall, according to the FDA's advisory. The risk arises during normal system use or when initiated by an operator in an emergency using the magnet emergency stop button.
The announcement emphasized that this is a "correction, not a product removal."
Here are the specifics on the 150 units that are impacted by the recall:
- Product Names: Panorama 1.0T HFO
- Product Codes: LNH
- Model Numbers: 781250 and 781350
- Distribution Dates: January 1, 2001 to October 1, 2016
- Devices Recalled in the U.S.: 150
- Date Initiated by Firm: November 20, 2023
In response to the recall, Philips recommends that affected healthcare providers cease using the Panorama 1.0T HFO systems immediately and follow the provided instructions for proper device disposition. The company sent a letter instructing healthcare organizations to do the following:
- Immediately discontinue use of any impacted MR system(s).
- Do not initiate a manual quench of the magnet, unless there is an emergency.
- Post a “do not use” notice on or near the impacted MR system(s)
- Circulate the “do not use notice” to all users of this device so that they are aware of the product issue and associated hazard/harm until this issue has been resolved.
- Complete and return the updated customer response form to Philips within 30 days.
The full recall notice can be read by clicking here.