PHOTO GALLERY: Examples of FDA-cleared AI in radiology
This is a photo gallery of artificial intelligence products that have been cleared for clinical use in medical imaging by the U.S. Food and Drug Administration. Radiology by far is the leader of all clinical AI FDA approvals, with 723 out of a total of 950 cleared algorithms as of July 2024.
This photo gallery includes images shot at recent medical conferences and from vendors showing examples of commercialized AI already in use for radiology.
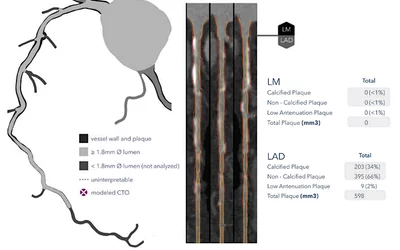
HeartFlow's AI Plaque Analysis software quantifies areas of soft plaque and differentiates the various types of plaque from cardiac CT imaging. It shows a 3D plaque model and analysis by territory across calcified, non-calcified and low-attenuation plaques. This includes viewing cross-sectional, color-coded images of each plaque type where it was quantified along the vessel. The AI was cleared by the FDA in October 2022.
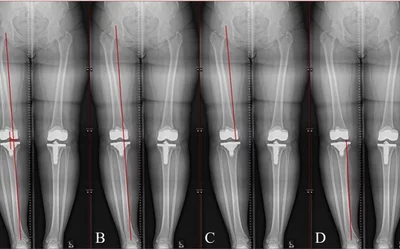
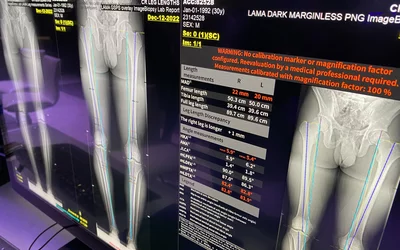
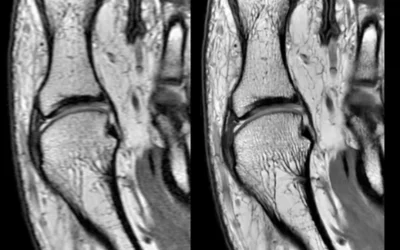
AI automated measurement of limb-length discrepancy and quantitative knee alignment parameters on uni- and bilateral AP full leg radiographs using the IB Lab LAMA algorithm. Artificial intelligence-assisted leg measurements are as accurate as those taken manually by radiologists, but are 90% faster than manual measurements. The AI was cleared in June 2023. Courtesy of Clinical Imaging
Artificial intelligence sign in the AI section of the United Imaging booth at the Radiological Society of North America 2023 meeting. The Chinese company wants to break into the U.S. market and has had 20 algorithms cleared by the FDA for use on its CT, MR, X-ray and ultrasound systems since 2020. There were 10 approvals just the past year. Photo by Dave Fornell.
Cleerly AI analysis for soft coronary plaques in the right coronary artery from coronary computed tomography angiography (CCTA) scans displayed at the American College of Cardiology (ACC) 2024 meeting. The ability of AI to quickly segment and accurately quantify soft plaques may be a paradigm shift in preventive cardiology for much earlier detection of pre-symptomatic disease, earlier start to statin medications and then serial monitoring of the impact of cholesterol-lowering medications. Cleerly's first of three FDA clearances was in 2019.
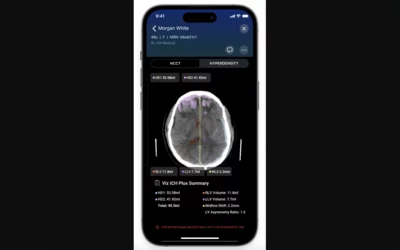
A few vendors offer alert systems where AI does a first pass of all images coming off a CT scanner and sends automated alerts if it detects a suspected stroke, pulmonary embolism, aortic aneurism or other emergency diagnosis that needs immediate attention. This is example shows the smart phone alert view of the Viz.ai ICH Plus algorithm. The AI obtains accurate and quantifiable measurements of intracerebral hemorrhages to immediately send to stroke teams before the exam has even entered the PACS system.
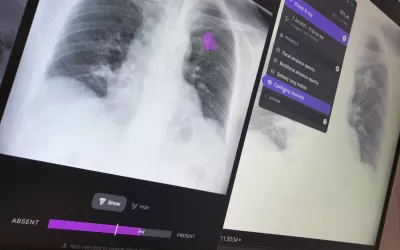
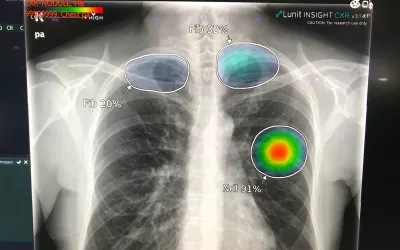
Many radiologists are still unsure about how much to trust artificial intelligence algorithms in reading exams. In response, some vendors have added confidence level indicators (bar graph under image) to show the level of certainty the AI has in its own finding based on the studies it was trained on. This is an AI lung assessment software from Annalise.AI at RSNA 2022, which gained FDA clearance in March 2023.
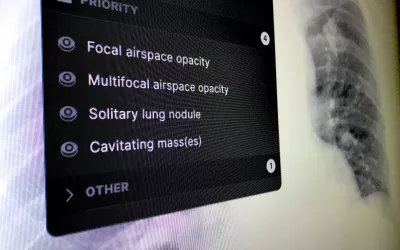
Example of artificial intelligence lung X-ray analysis detecting four different findings and marking them as priority in the reading list before the exam is seen by a radiologist, who can then confirm the findings. Example shown by Annalise.AI at RSNA 2022. This algorithm gained FDA clearance in March 2023.
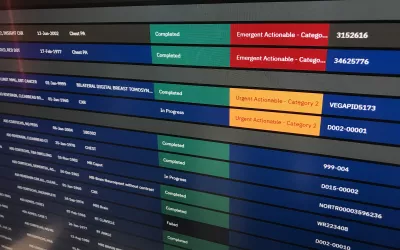
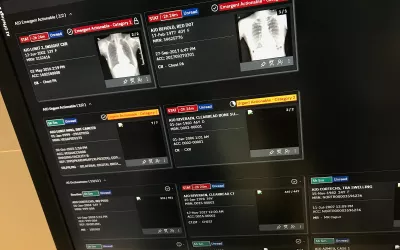
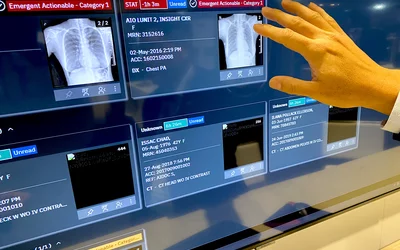
Example of AI sorting exams in a radiology DICOM worklist to read first based on the AI screening the studies and marking the ones that are not normal and likely to have critical findings at the top of the list for priority reads. Example shown by Merge/Merative at RSNA 2022. The technology was cleared by the FDA in October 2019. Similar technology exists on most PACS vendors software with elements of workflow orchestration.
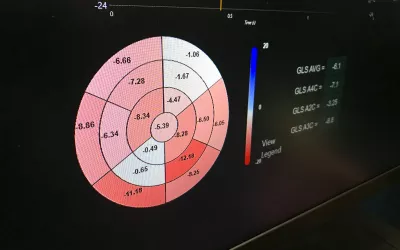
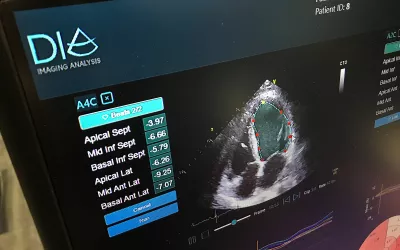
AI automated bullseye plot of strain deficits in the walls of the heart from a cardiac ultrasound exam on Dia's AI-enabled strain echocardiogram software. Dia gained FDA approval for its automated strain in December 2021. Dia is now owned by Philips Healthcare.
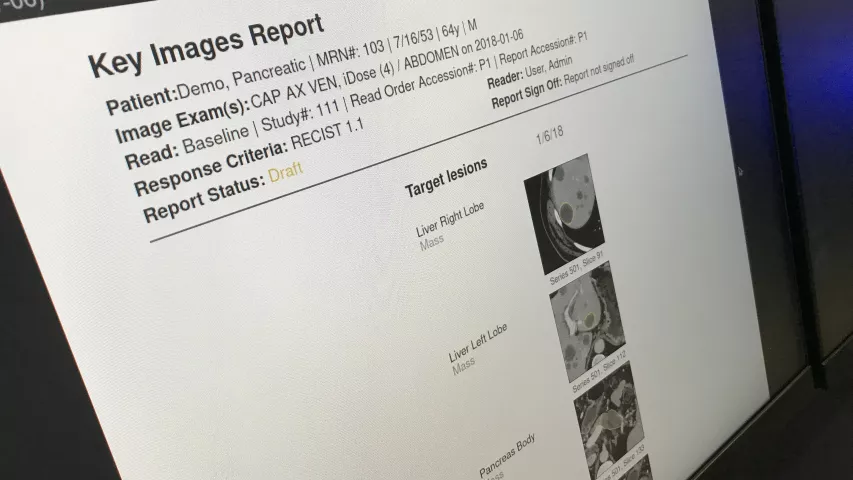
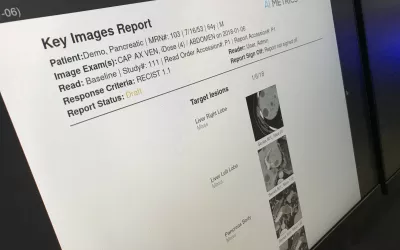
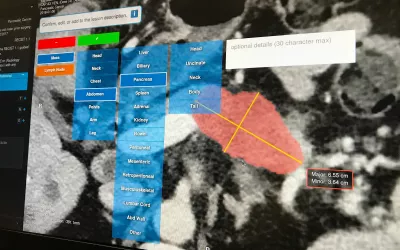
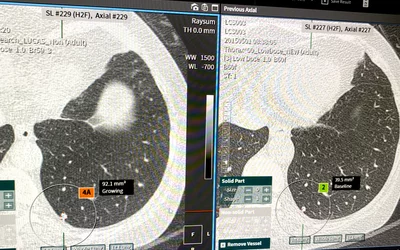
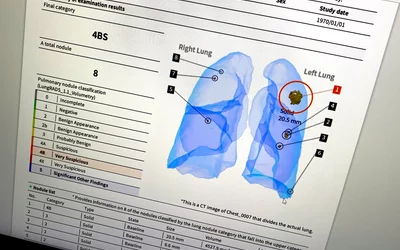
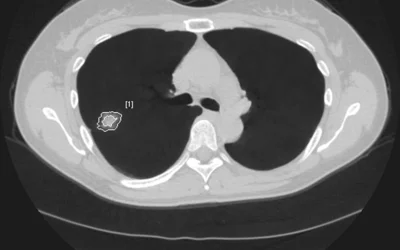
Automated lung cancer detection and contouring by the AI Metrics cancer followup RECIST analysis artificial intelligence. The software is designed to speed serial cancer imaging exams to determine if treatment is working or not. The AI also helps increase consistency in the quantification of the tumor. The AI was cleared by the FDA in December 2020.
Strain cardiac echo imaging has become a hot topic in cardiac ultrasound and many cardiologists now see utility with the additional clinical information it can provide to inform care decisions. However, it can be time consuming and many centers have low usage of strain, even if they have strain packages on their systems. Dia's automated strain AI application automates the process to increase strain usage. The AI gained FDA in December 2021. Dia is now owned by Philips Healthcare.
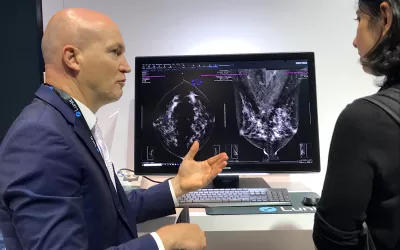
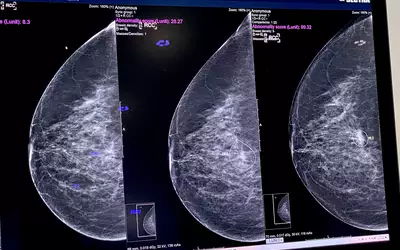
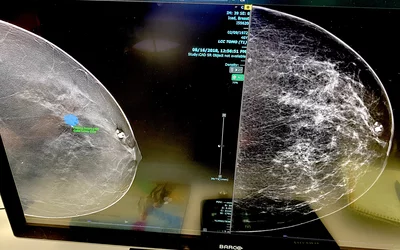
A product rep discussing breast automated detection AI in the crowded Lunit booth at RSNA 2022. The vendor's Insight MMG breast computer-assisted detection and diagnosis (CADe/x) AI is intended to aid in the detection, localization, and characterization of suspicious areas for breast cancer on mammograms. The FDA cleared it for FFDM in November 2021, and for DBT in November 2023. Photo by Dave Fornell at RSNA 2023.
Example of an AI-initiated acute care team alert on a smart phone for an abdominal aortic aneurysm (AAA) detection from a CT scan. The technology can speed diagnosis and treatment for these critical patients by sending automated alerts before the images are even loaded into the PACS. The AI gained FDA in March 2023.
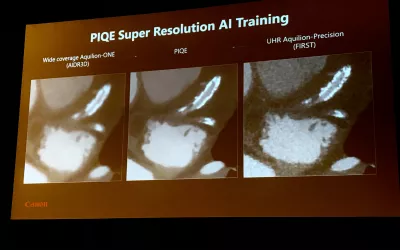
Example of an AI CT image reconstruction algorithm that can enhance imaging quality, reduce noise and reduce radiation dose. It also helps eliminate artifacts like metal and calcium blooming, as seen here, to see the lumen inside a coronary stent. This is Canon's second generation reconstruction AI, which was gained FDA in March 2024.
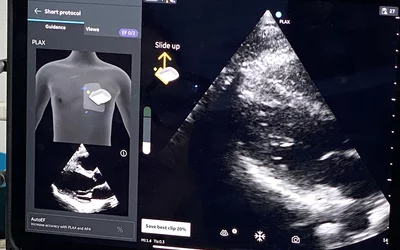
Performing a high quality cardiac ultrasound exam can take a lot of training and experience, but several vendors now offer AI-guidance for echo exams that can greatly improve the diagnostic quality of novice exams, especially with POCUS. This is the Caption Health AI integrated with the GE Vscan at the American College of Cardiology (ACC) 2024 meeting. The AI shows on the screen how and where to hold the transducer and offers other instructions to capture the exam. The AI was cleared in April 2020.
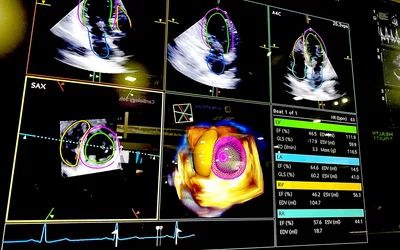
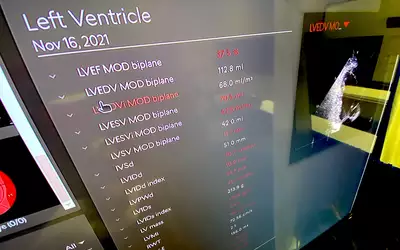
Automated strain cardiac ultrasound analysis performed by AI that chose from a 3D echo exam the best views and points in the cardiac cycle to make measurements and create consistently reproducible numbers on the new Siemens Acuson Origin system. The Origin provides more than 5,600 real-time, AI-powered measurements trained on 2 billion images. It can perform automated ventricular volumes on contrast and noncontrast acquisitions. Key measures span across all four chambers and quantification in M-mode, 2D, spectral and tissue Doppler and without ECG gated TEE imaging. It was cleared in October 2023. Read more
Radiology workflow automated AI reporting in the GE Healthcare PACS using partner software IB Lab LAMA orthopedic imaging measurements and quanitification, displayed at RSNA 2023. The AI gained FDA clearance in June of 2023. Many PACS vendors have partnered with AI vendors to enable closer integration and because they realized it was not possible to develop AI software for every use case in-house.
AI-enabled patient isocentering system seen on the rear screen of GE Omni Legend PET/CT nuclear imaging system at RSNA 2023. This auto positions the patient on the table to get the best quality imaging so the technologist does not need to spend extra time on patient set up. This AI was cleared by the FDA in January 2020. Photo by Dave Fornell.
AI-enabled patient isocentering system showing an alert that the patient needs to face a different direction for the imaging protocol on the GE Omni Legend PET/CT nuclear imaging system at RSNA 2023. These types of AI alerts for the technologist can save time and prevent rescanning or recalling patients for another scan due to poor diagnostic image quality. This AI was cleared by the FDA in January 2020. Photo by Dave Fornell.
The overhead AI-enabled patient isocentering gantry of the GE Revolution Apex CT system at RSNA 2023. The overhead camera detects the patient and the table and can automatically determine if a patient needs to be repositioned, or can recenter the table to enable optimal scan image quality. AI cleared in January 2020. Photo by Dave Fornell.
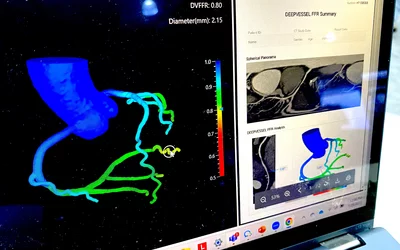
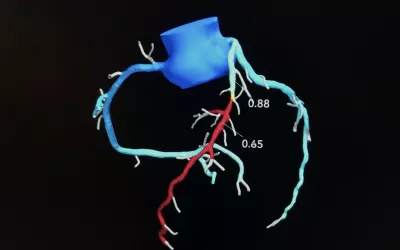
AI fractional flow reserve derived from computed tomography (FFR-CT) DeepVessel FFR AI reconstruction from a cardiac CT scan demonstrated by Keya Medical at the Society of Cardiovascular Computed Tomography (SCCT) 2023 meeting. Keya is one of at least three vendors on the U.S. market that now offer FFR-CT to better assess coronary disease than CT alone. The AI was cleared by the FDA in April 2022.
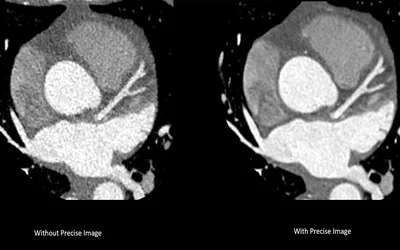
A comparison showing the improved image quality using the Precise Image AI reconstruction software on a Philips Healthcare CT 5300 scanner. The AI was cleared in January 2022. All major CT vendors are moving to AI-based image reconstruction algorithms to enable diagnostic quality, low radiation dose scans and reduce or eliminate calcium and metal blooming artifacts.
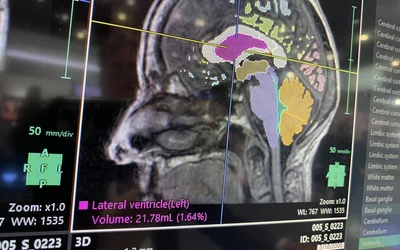
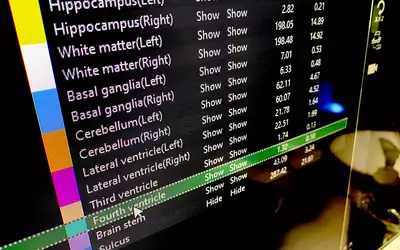
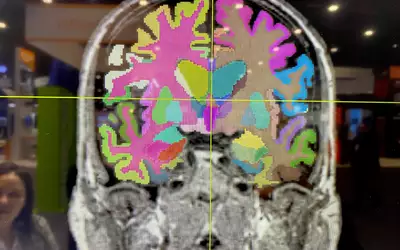
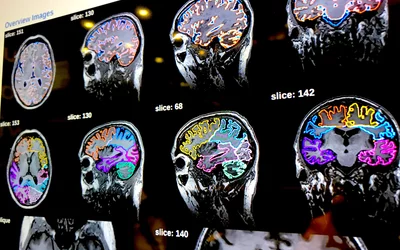
Brain MRI automated AI anatomical segmentation and measurements on Fujifilm's Synapse 3D system displayed at RSNA 2023. The automation of complex measurements can help free up radiologists to concentrate on diagnosis and report, rather than spending a sizable amount of time using manual calipers. This AI was cleared in January 2021. Photo by Dave Fornell.
Brain MRI automated AI measurements on Fujifilm's Synapse 3D system displayed at RSNA 2023. The automation of complex measurements can help free up radiologists to concentrate on the diagnosis and report, rather than spending a sizable amount of time using manual calipers. This AI was cleared in January 2021. Photo by Dave Fornell.
Entry way into the Radiological Society of North America (RSNA) artificial intelligence showcase on the expo floor, where more than 200 vendors offered commercialized AI for imaging. Radiology has the highest number of FDA-cleared algorithms, making RSNA the largest clinical AI conference in the United States. Photo by Dave Fornell.
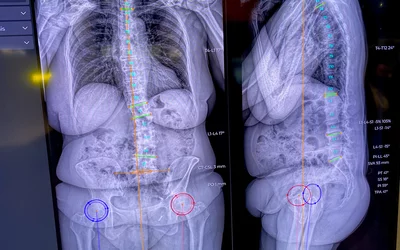
Analysis and automated measurements of spinal deformities, degenerative diseases, lower limb alignment disorders, and deformities through precise angle and length measurements using the VEA Align AI on the EOS load-bearing X-ray imaging system displayed at RSNA 2023. The cloud-based AI software received FDA clearance in January 2024. Photo by Dave Fornell.
The Lunit Insight MMG computer-assisted detection and diagnosis (CADe/x) AI software is intended to aid in the detection, localization, and characterization of suspicious areas for breast cancer on mammograms. The FDA cleared the AI for full-field digital mammography in November 2021, and for 3D breast tomosynthesis in November 2023. Lunit's lesion detection AI with its certainty score shown on the Sectra PACS platform. Photo by Dave Fornell at RSNA 2023.
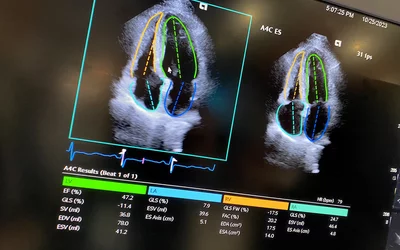
Example of automated AI cardiac ultrasound measurements on the Siemens Acuson Origin cardiac ultrasound system, cleared by the FDA in October 2023. The AI has automatic view recognition and anatomical contouring to pick the best view and position to make more than 5,000 types of measurements and automate strain and ejection fraction assessments.
US AI automated measurements for use in an echocardiogram lab, or using a point-of-care ultrasound (POCUS) system to standardize cardiac ultrasound measurements. Reproducibility in measurements varies greatly between sonographers depending on their level of experience and how and where calipers are placed, and during which portion of the cardiac cycle. AI is being increasingly used in ultrasound to make measurements more consistent and meaningful, especially between serial exams of the same patient. Example from ASE 2023 meeting. Algorithm first gained FDA in November 2019.
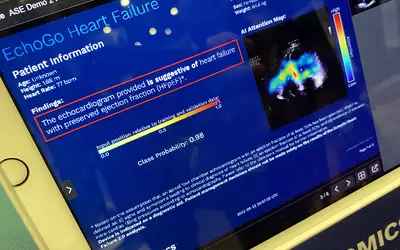
An AI generated report that detects heart failure patients with preserved ejection fraction (HFpEF) by looking at a single apical four-chamber view during a cardiac ultrasound exam. HFpEF can be difficult to detect on echocardiography, and this is the first AI cleared to detect it, filling an unmet need. The Ultromics EchoGo technology received the FDA's breakthrough device designation and was then cleared by the FDA at the end of 2022. Photo by Dave Fornell at the American Society of Echo (ASE) 2023 meeting.
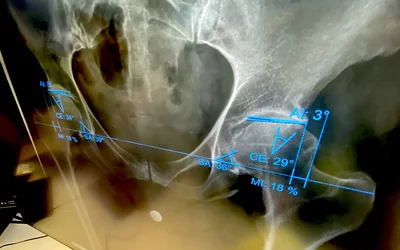
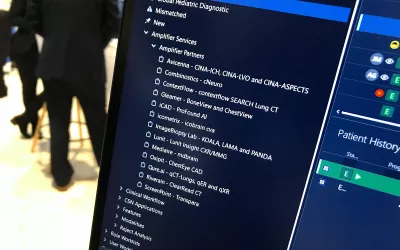
AI automated measurements for hip dysplasia using a third-party AI software on the Sectra PACS system. The vendor offers its Sectra Amplifier Marketplace, where there are currently 60 FDA cleared AI algorithms their PACS users can purchase that will integrate seamlessly with the Sectra system. Example of AI shown at HIMSS 2023. Photo by Dave Fornell.
Nuance has taken traditional radiology dictation to a new level with its PowerScribe One that uses generative AI to provide workflow enhancements to help radiologists automate reports and increase accuracy. However, AI integrated into radiology reporting systems generally does not need FDA clearance because it is not directly clinically involved in the diagnosis or treatment of a patient. This example shown at HIMSS 23 is among hundreds, if not thousands, of AI algorithms in healthcare IT systems that do not currently require regulation by the FDA. That may change in the future as the FDA is now asking IT companies to look more closely at their AI to see if they have a direct impact on care.
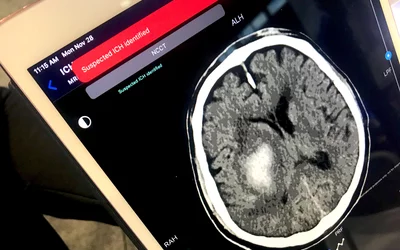
Example of an artificial intelligence-automated notification on an iPad alerting a stroke team that an intracranial hemorrhage has been detected. These alerts can help speed review by a radiologist and treatment for the patients with a head CT showing ICH and CT head large vessel occlusion (LVO). This was demonstrated using Terarecon's software at RSNA 2022. The AI was cleared in November 2022. Photo by Dave Fornell.
Assessment of brain atrophy on MRI using the automated Quantib Neurodegenerative AI algorithm, which gained FDA in January 2022. This example was displayed at RSNA 2022.
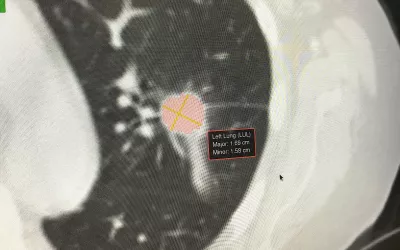
Example of pulmonary vessel suppression AI in a chest CT to help radiologists better identify and assess lung nodules using the Riverain ClearRead CT, which was cleared in September 2016. The AI to suppress the vessels was cleared based on the predicated Riverain DeltaView AI for bone suppression in chest X-rays, which was cleared by the FDA in December 2011.
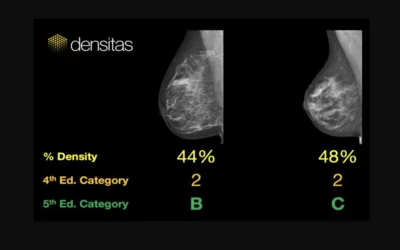
An example of commercially available artificial intelligence-automated grading of breast density on mammograms from the vendor Densitas. The Densitas densityai software is compatible with FFDM and digital breast tomosynthesis systems and provides an ACR BI-RADS Atlas 5th Edition breast density category to aid interpreting physicians in the assessment of breast tissue composition. It was first cleared in February 2018.
Incorporating AI from numerous vendors can be a major headache for IT departments. Recognizing this, numerous large PACS vendors now offer seamless integration with many third-party AI vendors. This is an example of the seamless integration to AI partners on the Sectra enterprise imaging system. Radiologists can choose AI from the Sectra partner list that is fully integrated into the workflow on the system without requiring extra logins, different screens or workstations. Photo by Dave Fornell RSNA 2022.
Performing CT-derived fractional flow reserve (FFR-CT) improves the accuracy of coronary CT angiography (CCTA) and helps limit unneeded invasive coronary angiography. It creates a coronary tree from a CT scan and the vessels are color coded based on the drops in blood flow derived by computation fluid dynamics AI. It is the first AI algorithm to be included in any cardiology guidelines. It was first cleared in January 2016.
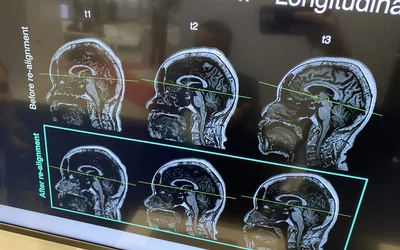
AI reformatting of misaligned patients in the MRI scanner by Subtle Medical's SubtleALLY demonstrated at RSNA 2023. This slight change in formatting may be important for patients undergoing serial exams used in cancer treatments or disease monitoring. The AI was cleared in May 2023. Photo by Dave Fornell.
Related Radiology AI Content:

Dave Fornell has covered healthcare for more than 17 years, with a focus in cardiology and radiology. Fornell is a 5-time winner of a Jesse H. Neal Award, the most prestigious editorial honors in the field of specialized journalism. The wins included best technical content, best use of social media and best COVID-19 coverage. Fornell was also a three-time Neal finalist for best range of work by a single author. He produces more than 100 editorial videos each year, most of them interviews with key opinion leaders in medicine. He also writes technical articles, covers key trends, conducts video hospital site visits, and is very involved with social media. E-mail: dfornell@innovatehealthcare.com