AI company racks up 7 new FDA clearances for image triage and notification solutions
The U.S. Food and Drug Administration just granted Annalise.ai seven additional 510(k) clearances for the company’s suite of AI-assisted triage and notification solutions.
The Australia-based company made the announcement on April 12 in a release that described the timing of these AI-assisted solutions as “increasingly important” amid growing workloads and staffing shortages.
“Triage solutions drive quality improvement by enabling earlier detection and intervention for our most critically ill patients. And by prioritizing the radiology work list, these solutions also help address some of the burnout issues that have affected our specialty since the pandemic,” Rick Abramson, MD, Annalise.ai’s chief medical officer, said a prepared statement.
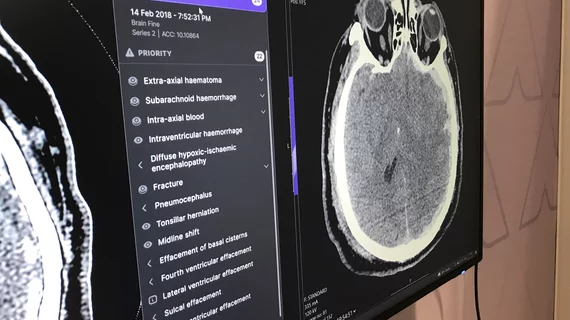
The newest clearances pertain to the company’s triage notification software, which utilizes deep learning to identify time sensitive, critical pathologies on imaging and flag them as priority cases on radiologist work lists. Specifically, the clearances fall under Annalise.ai’s Triage CT Brain and Triage Chest X-ray offerings.
These latest approvals bring Annalise.ai’s total of FDA-cleared findings to nine.
Triage CT Brain software can flag the following four critical findings:
Acute subdural/epidural hematoma
Acute subarachnoid hemorrhage
Intra-axial hemorrhage
Intraventricular hemorrhage
While Triage Chest X-Ray offers a total of five time sensitive findings:
Pneumothorax
Tension pneumothorax
Pleural effusion
Pneumoperitoneum
Vertebral compression fracture
Lakshmi Gudapakkam, CEO of Annalise.ai, also expressed enthusiasm for the company’s growing presence in the U.S. market following its latest FDA clearances.
“We are thrilled to mark our entry into the U.S. neuroimaging space with this set of high-precision tools for prioritizing intracranial hemorrhage cases on noncontrast head CT,” Gudapakkam said in a statement. “Annalise.ai is committed to developing the highest quality tools for advancing patient care and patient care efficiency in the U.S.”
Annalise.ai received its first FDA clearance in February of 2022. That approval was for the first AI product available in the U.S. that could differentiate a tension pneumothorax on chest X-ray.
To learn more, click here.