AI brain imaging device receives FDA clearance
South Korean startup Vuno’s AI-powered brain imaging device has received the 510(k) certification from the U.S. Food and Drug Administration (FDA), signaling the company’s expansion into U.S. markets where it hopes to improve the early diagnosis of dementia.
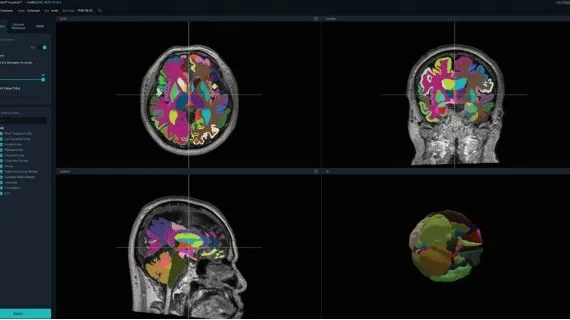
The Vuno Med-DeepBrain automatically enhances MRI images, segmenting the brain into 100 distinct areas to identify and measure any reduction in brain activity almost immediately. The technology is designed to assist clinicians in the diagnosis of neurodegenerative conditions and dementia, including Alzheimer’s, providing more imaging data than what’s possible with just an MRI.
In a statement, Vuno says its DeepBrain AI has the potential to identify those at risk for dementia, such as when a patient is in “subjective cognitive decline,” meaning they’ve yet to show signs of serious illness. DeepBrain highlights areas of concern in the brain and offers a potential diagnosis based on its measurements of brain function.
“With the recent advent of next-generation dementia treatments, there is a growing demand for early diagnosis, and we will strengthen our sales efforts to rapidly expand this product in the U.S. market and contribute to solving the problem of dementia,” Lee Ye-ha, Vuno’s CEO said in the statement.
In July, Vuno showcased the Med-DeepBrain’s capabilities at the Alzheimer's Association International Conference in Amsterdam, presenting a clinical study that demonstrated the ability of the device to diagnose Alzheimer in the early stages of the disease.
In the study, Vuno’s AI was able to outperform medical professionals in the identification of normal cognition and signs of Alzheimers from the review of brain MRIs from 98 elderly patients.