Oncology Imaging
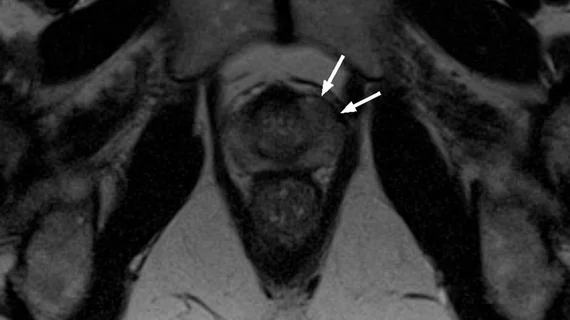
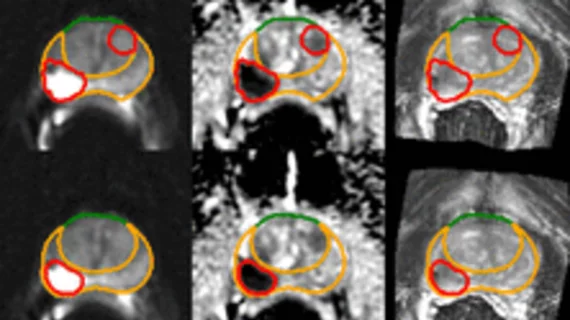
Medical imaging has become integral to cancer care, assessing the stage and location of cancerous tumors. By utilizing powerful imaging modalities including CT, MRI, MRA and PET/CT, oncology imaging radiologists are able to assist referring physicians in the detection and diagnosis of cancer.
Displaying 1161 - 1168 of 2554